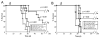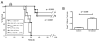In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy - PubMed (original) (raw)
In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy
James C Lee et al. Cancer Res. 2011.
Abstract
Human T cells genetically modified to express chimeric antigen receptors (CAR) specific to the B cell tumor antigen CD19 can successfully eradicate systemic human CD19(+) tumors in immunocompromised SCID (severe combined immunodeficient)-Beige mice. However, in the clinical setting, CD4(+) CD25(hi) T regulatory cells (Treg) present within the tumor microenvironment may be potent suppressors of tumor-targeted effector T cells. In order to assess the impact of Tregs on CAR-modified T cells in the SCID-Beige xenotransplant model, we isolated, genetically targeted and expanded natural T regulatory cells (nTreg). In vitro nTregs modified to express CD19-targeted CARs efficiently inhibited the proliferation of activated human T cells, as well as the capacity of CD19-targeted 19-28z(+) effector T cells to lyse CD19(+) Raji tumor cells. Intravenous infusion of CD19-targeted nTregs into SCID-Beige mice with systemic Raji tumors traffic to sites of tumor and recapitulate a clinically relevant hostile tumor microenvironment. Antitumor efficacy of subsequently infused 19-28z(+) effector T cells was fully abrogated as assessed by long-term survival of treated mice. Optimal suppression by genetically targeted nTregs was dependent on nTreg to effector T-cell ratios and in vivo nTreg activation. Prior infusion of cyclophosphamide in the setting of this nTreg-mediated hostile microenvironment was able to restore the antitumor activity of subsequently infused 19-28z(+) effector T cells through the eradication of tumor-targeted nTregs. These findings have significant implications for the design of future clinical trials utilizing CAR-based adoptive T-cell therapies of cancer.
©2011 AACR.
Figures
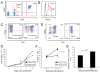
Figure 1. Efficient transduction and expansion of CAR+ nTregs
(A) FACS analysis of nTregs isolated from peripheral blood utilizing the Dynal Regulatory T Cell Isolation Kit (Invitrogen). (B) FACS analyses of isolated nTregs as assessed by intracellular staining for Foxp3 expression. Blue line represents isotype control, green line represents CD4+ CD25- non-Treg control and red line represents nTregs. (C) FACS analyses for Foxp3 and 19z1 expression of isolated nTregs at day 7 following isolation and 19z1 CAR retroviral gene transfer. Similar gene transfer was obtained for nTregs transduced with the 19-28z and Pz1 control CARs (data not shown). (D) nTreg expanded following activation with Dynal CD3/CD28 Human Treg Expander beads (day 0), transduction with the 19z1 CAR, and restimulation (arrow, day 14) with either Expander beads or 3T3(hCD19/CD80) AAPCs. Cell counts, normalized to total cell number, show similar expansion between the AAPC and CD3/CD28 bead activated groups. (E) Foxp3 expression is largely retained following restimulation with either Dynal CD3/CD28 Human Treg Expander beads or 3T3(hCD19/CD80) AAPCs, with the latter population demonstrating enhanced CAR expression. (F) Percentage of expanded T cells expressing the CAR is increased following stimulation on 3T3(hCD19/CD80) AAPCs, but stable following stimulation with Dynal CD3/CD28 Human Treg Expander beads (p < 0.01). (G) Absolute numbers of 19z1+ Tregs is increased following expansion on 3T3(hCD19/CD80) AAPCs when compared to expansion with Dynal CD3/CD28 Human Treg Expander beads.
Figure 2. CAR+ nTregs inhibit expansion of activated naïve T cells, and cytotoxicity of 19-28z+ effector T cells
(A) CSFE-labeled naïve T cells co-cultured with 19z1+ nTregs, Pz1+ nTregs, or 19z1+ Foxp3- CD4+ CD25- T cells at titrated effector to suppressor ratios were activated with CD3/CD28 Human T cell Expander beads. CFSE+ T cells were analyzed via flow cytometry on day 3 post activation. Percent proliferation was calculated using FlowJo software. 19z1+ and Pz1+ nTregs, in contrast to control non-Tregs, efficiently suppressed proliferation of naïve T cells. (B) 19z1+ nTregs inhibit secretion of IL-2 by activated naïve T cells in a dose dependent manner, as assessed by Luminex-based analyses of tissue culture supernatants at 24 hours post co-culture. (C) CFSE-labeled 19-28z+ effector T cells co-cultured with 19z1+ nTregs (solid bars) or Foxp3- CD4+ CD25- T cells at varying effector to suppressor ratios were activated on 3T3(hCD19/CD80) AAPCs for 3 days. 19z1+ nTregs, but not control non-Tregs, suppressed proliferation of 19-28z+ effector T cells. (D) 19z1+ nTregs inhibit 19-28z+ effector T cell cytotoxicity. CD19+ Raji tumor cells were co-cultured at a 1:1:1 ratio with 19-28z+ effector T cells and 19z1+ nTregs. At 24 hours, persistence of Raji tumor cells was assessed by FACS with corresponding FSC/SSC plots provided. Persistence of Raji tumor cells is evident when 19-28z+ effector T cells were co-cultured with 19z1+ nTregs, in contrast to coculture with 19-28z+ effector T cells in the absence of nTregs. (E) Standardized cytotoxicity assay of CD19+ Raji tumor cells co-cultured with 19-28z+ effector T cells alone or 19-28z+ effector T cells with 19z1+ nTregs (at a 1:1 ratio) at various effector to target (E:T) ratios. E:T ratios represent the ratio of 19-28z+ effector T cells to target Raji tumor cells.
Figure 3. CD19-targeted CAR+ nTregs traffic to CD19+ Raji tumor cells in vivo
(A) Dual bioluminescent imaging of Raji tumor and CAR+ nTregs show trafficking of 19z1+ nTregs, but not Pz1+ nTregs, to subcutaneous Raji tumors at 24 hours. SCID-Beige mice were injected subcutaneously with Raji(GFP-FFLuc) cells and 10 days later, with 19z1+ extGLuc+ or control Pz1+ extGLuc+ nTregs. Tumor cells were imaged using the FFLuc specific luciferin substrate, while T cells were imaged using the GLuc specific coelenterazine substrate. (B) Immunohistochemistry staining with an anti-human CD3 antibody confirms the presence of 19z1+ nTregs, but not Pz1+ nTregs, within Raji tumor microenvironment. (C) Bioluminescent imaging CAR+ nTregs show trafficking of 19z1+ nTregs, but not Pz1+ nTregs, to systemic Raji tumors at 24 hours, with predicted signal in the bone marrow of the femurs, tibia, and humeri (green arrows) of 19z1+ extGluc+ nTreg infused mice, as well as infiltration of these nTregs into the submandibular lymphnodes (orange arrow).
Figure 4. CD19-targeted nTregs within the Raji tumor microenvironment suppress 19-28z+ effector T cell function in vivo
(A) SCID-Beige mice were injected i.v. with Raji tumor cells on day 0, followed by CAR+ nTregs on day 5 (filled arrow) and CAR+ effector T cells on day 6 (open arrow). 19z1+ nTregs fully abrogated eradication of systemic Raji tumors by 19-28z+ effector T cells as assessed by survival over time when compared to mice treated with 19-28z+ effector T cells alone (p < 0.001). Pz1+ nTregs did not demonstrate significant suppression (p = 0.09 when compared to the 19-28z+ effector T cells alone cohort; p < 0.001 compared to the 19z1+ nTreg plus 19-28z+ effector T cells cohort). Data represents combined results from 2 independent experiments. (B) 19z1+ nTregs inhibited effector 19-28z+ T cells in a dose dependent manner with infused nTreg to effector T cell ratios of 1:1, 1:4, and 1:8 resulting in no long term surviving mice (all with p < 0.001, compared to 19-28z Teff alone cohort) while a 1:16 nTreg to effector T cell ratio allowed for a 50% long-term survival of treated mice (p = 0.02, compared to Pz1+ Teff treated control cohort). Survival of the 1:16 nTreg to effector T cell treated cohort was statistically similar to the 19-28z Teff alone control cohort (p = 0.3). Similar results were obtained in tumor bearing mice following prior infusion with 19-28z+ nTregs (data not shown). d: days since Raji tumor cell injection.
Figure 5. Optimal in vivo nTreg suppression is dependent on activation of nTregs within the tumor microenvironment
(A) Schematic of the 19(del) CAR. Black box, CD8 leader sequence; green box, (Gly3Ser)4 linker; LTR, long terminal repeat; SD, splice donor; SA, splice acceptor; arrows, start of transcription. (B) 19(del)+ nTregs, like control Pz1+ nTregs, but in contrast to 19z1+ nTregs, fail to expand following co-culture on 3T3(hCD19/CD80) AAPCs consistent with a lack of T cell activation mediated through the 19(del) CAR. (C) 19(del)+ extGLuc + nTregs retain the ability to traffic to Raji tumor in vivo. SCID-Beige mice bearing palpable Raji(GFP-FFLuc) tumors were injected i.v. with 19(del)+ extGLuc+ T cells. Mice were imaged at 24 hours post nTreg infusion. (D) 19(del) + nTregs retain the ability to inhibit expansion of activated naïve T cells. CFSE-labeled naïve T cells co-cultured with 19(del) + Tregs, 19z1+ Tregs, or 19z1+ CD4+ CD25- non-Treg control T cells at varying effector to suppressor ratios were activated with Dynabeads CD3/CD28 Human T cell Expander beads. Both 19(del)+ nTregs and 19z1+ nTregs suppressed proliferation of naïve T cells when compared to the non-Treg control co-cultures. (E) Infusion of Raji tumor bearing mice with 19(del)+ nTregs partially inhibited antitumor efficacy of 19-28z+ effector T cells as assessed by long-term survival. Survival of mice previously infused with 19(del)+ nTregs, when compared to mice treated with 19-28z+ effector T cells alone, was significantly lower (p < 0.001) but significantly improved when compared to mice previously treated with 19z1+ nTregs (p < 0.001). Data represents combined results from 2 independent experiments. Closed arrow: nTreg infusion; open arrow, effector 19-28z+ T cell infusion; d, days since Raji tumor cell injection. At 24 hours following 19-28z+ effector T cell infusion, prior infusion with either 19z1+ or 19(del)+ nTregs significantly reduced serum levels of IL-2 (F) (p < 0.005), and IFNγ (G) (p < 0.05), when compared to mice treated with 19-28z+ effector T cells alone. Figure 5F and 5G represent the combined data from 2 independent experiments with cohorts of 3 mice in each experiment.
Figure 6. Cyclophosphamide lymphodepletion following 19z1+ nTreg infusion, enhances 19-28z+ effector T cell tumor cell eradication, altering the nTreg to effector T cell ratio within the tumor microenvironment
(A) SCID-Beige were mice injected i.v. with Raji tumor cells on day 0, followed by 19z1+ nTregs on day 5 (left filled arrow), i.p. injection of cyclophosphamide (100mg/kg) on day 6 (open arrow), and 19-28z or Pz1+ effector T cells on day 7 (right black arrow). Long term survival comparable to no-nTreg controls was observed in mice injected with 19z1+ nTregs followed by cyclophosphamide therapy and 19-28z+ effector T cell infusion which was significantly superior to similarly treated mice without prior lymphodepletion (p < 0.0001). Data represents combined results from 2 independent experiments. (B) Foxp3+ nTreg to effector cell ratios were assessed in tumor-involved tissues (bone marrow) by FACS at 24 hours following 19-28z+ effector T cell infusion. Prior cyclophosphamide therapy significantly altered the nTreg to effector T cell ratios in favor of the 19-28z+ effector T cells (0.14 with versus 0.9 without lymphodepletion, p < 0.005). Data represent the average from 2 independent experiments each with cohorts of 3 mice per treatment group.
Similar articles
- Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts.
Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, Quintás-Cardama A, Larson SM, Sadelain M. Brentjens RJ, et al. Clin Cancer Res. 2007 Sep 15;13(18 Pt 1):5426-35. doi: 10.1158/1078-0432.CCR-07-0674. Epub 2007 Sep 12. Clin Cancer Res. 2007. PMID: 17855649 - A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines.
Schneider D, Xiong Y, Wu D, Nӧlle V, Schmitz S, Haso W, Kaiser A, Dropulic B, Orentas RJ. Schneider D, et al. J Immunother Cancer. 2017 May 16;5:42. doi: 10.1186/s40425-017-0246-1. eCollection 2017. J Immunother Cancer. 2017. PMID: 28515942 Free PMC article. - Fully human CD19-specific chimeric antigen receptors for T-cell therapy.
Sommermeyer D, Hill T, Shamah SM, Salter AI, Chen Y, Mohler KM, Riddell SR. Sommermeyer D, et al. Leukemia. 2017 Oct;31(10):2191-2199. doi: 10.1038/leu.2017.57. Epub 2017 Feb 16. Leukemia. 2017. PMID: 28202953 Free PMC article. - Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors.
Kochenderfer JN, Rosenberg SA. Kochenderfer JN, et al. Nat Rev Clin Oncol. 2013 May;10(5):267-76. doi: 10.1038/nrclinonc.2013.46. Epub 2013 Apr 2. Nat Rev Clin Oncol. 2013. PMID: 23546520 Free PMC article. Review. - Chimeric antigen receptor therapy for chronic lymphocytic leukemia: what are the challenges?
Davila ML, Brentjens R. Davila ML, et al. Hematol Oncol Clin North Am. 2013 Apr;27(2):341-53. doi: 10.1016/j.hoc.2012.12.004. Hematol Oncol Clin North Am. 2013. PMID: 23561477 Free PMC article. Review.
Cited by
- Chimeric Antigen Receptor- and TCR-Modified T Cells Enter Main Street and Wall Street.
Barrett DM, Grupp SA, June CH. Barrett DM, et al. J Immunol. 2015 Aug 1;195(3):755-61. doi: 10.4049/jimmunol.1500751. J Immunol. 2015. PMID: 26188068 Free PMC article. Review. - Gene-engineered T cells for cancer therapy.
Kershaw MH, Westwood JA, Darcy PK. Kershaw MH, et al. Nat Rev Cancer. 2013 Aug;13(8):525-41. doi: 10.1038/nrc3565. Nat Rev Cancer. 2013. PMID: 23880905 Review. - Constitutively active MyD88/CD40 costimulation enhances expansion and efficacy of chimeric antigen receptor T cells targeting hematological malignancies.
Collinson-Pautz MR, Chang WC, Lu A, Khalil M, Crisostomo JW, Lin PY, Mahendravada A, Shinners NP, Brandt ME, Zhang M, Duong M, Bayle JH, Slawin KM, Spencer DM, Foster AE. Collinson-Pautz MR, et al. Leukemia. 2019 Sep;33(9):2195-2207. doi: 10.1038/s41375-019-0417-9. Epub 2019 Feb 28. Leukemia. 2019. PMID: 30816327 Free PMC article. - Impact of the selective A2AR and A2BR dual antagonist AB928/etrumadenant on CAR T cell function.
Seifert M, Benmebarek MR, Briukhovetska D, Märkl F, Dörr J, Cadilha BL, Jobst J, Stock S, Andreu-Sanz D, Lorenzini T, Grünmeier R, Oner A, Obeck H, Majed L, Dhoqina D, Feinendegen M, Gottschlich A, Zhang J, Schindler U, Endres S, Kobold S. Seifert M, et al. Br J Cancer. 2022 Dec;127(12):2175-2185. doi: 10.1038/s41416-022-02013-z. Epub 2022 Oct 20. Br J Cancer. 2022. PMID: 36266575 Free PMC article. - The basic principles of chimeric antigen receptor design.
Sadelain M, Brentjens R, Rivière I. Sadelain M, et al. Cancer Discov. 2013 Apr;3(4):388-98. doi: 10.1158/2159-8290.CD-12-0548. Epub 2013 Apr 2. Cancer Discov. 2013. PMID: 23550147 Free PMC article. Review.
References
- Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. - PubMed
- Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–86. - PubMed
- Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13:5426–35. - PubMed
- Cheadle EJ, Gilham DE, Hawkins RE. The combination of cyclophosphamide and human T cells genetically engineered to target CD19 can eradicate established B-cell lymphoma. Br J Haematol. 2008;142:65–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA086438/CA/NCI NIH HHS/United States
- K08 CA095152/CA/NCI NIH HHS/United States
- R25 CA096945/CA/NCI NIH HHS/United States
- R01 CA138738/CA/NCI NIH HHS/United States
- CA95152/CA/NCI NIH HHS/United States
- CA86438/CA/NCI NIH HHS/United States
- CA096945/CA/NCI NIH HHS/United States
- P01 CA059350/CA/NCI NIH HHS/United States
- CA08748/CA/NCI NIH HHS/United States
- P01 CA094060/CA/NCI NIH HHS/United States
- CA059350/CA/NCI NIH HHS/United States
- CA094060/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 CA138738-01/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- CA138738/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous