Lack of de novo phosphatidylinositol synthesis leads to endoplasmic reticulum stress and hepatic steatosis in cdipt-deficient zebrafish - PubMed (original) (raw)
Lack of de novo phosphatidylinositol synthesis leads to endoplasmic reticulum stress and hepatic steatosis in cdipt-deficient zebrafish
Prakash C Thakur et al. Hepatology. 2011 Aug.
Abstract
Hepatic steatosis is the initial stage of nonalcoholic fatty liver disease (NAFLD) and may predispose to more severe hepatic disease, including hepatocellular carcinoma. Endoplasmic reticulum (ER) stress has been recently implicated as a novel mechanism that may lead to NAFLD, although the genetic factors invoking ER stress are largely unknown. During a screen for liver defects from a zebrafish insertional mutant library, we isolated the mutant cdipthi559Tg/+ (hi559). CDIPT is known to play an indispensable role in phosphatidylinositol (PtdIns) synthesis. Here we show that cdipt is expressed in the developing liver, and its disruption in hi559 mutants abrogates de novo PtdIns synthesis, resulting in hepatomegaly at 5 days postfertilization. The hi559 hepatocytes display features of NAFLD, including macrovesicular steatosis, ballooning, and necroapoptosis. Gene set enrichment of microarray profiling revealed significant enrichment of endoplasmic reticulum stress response (ERSR) genes in hi559 mutants. ER stress markers, including atf6, hspa5, calr, and xbp1, are selectively up-regulated in the mutant liver. The hi559 expression profile showed significant overlap with that of mammalian hepatic ER stress and NAFLD. Ultrastructurally, the hi559 hepatocytes display marked disruption of ER architecture with hallmarks of chronic unresolved ER stress. Induction of ER stress by tunicamycin in wild-type larvae results in a fatty liver similar to hi559, suggesting that ER stress could be a fundamental mechanism contributing to hepatic steatosis.
Conclusion: cdipt-deficient zebrafish exhibit hepatic ER stress and NAFLD pathologies, implicating a novel link between PtdIns, ER stress, and steatosis. The tractability of hi559 mutant provides a valuable tool to dissect ERSR components, their contribution to molecular pathogenesis, and evaluation of novel therapeutics of NAFLD.
Copyright © 2011 American Association for the Study of Liver Diseases.
Figures
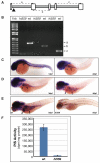
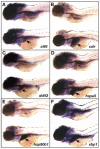
Figure 1. hi559 larvae exhibit defects in liver development
(A) 5-dpf larval morphology**,** wild-type on top, mutant below. (B) hi559 larvae show globular liver (yellow) and smaller intestine (red). (C) Globular liver is apparent by Cy3-SA labeling. Lateral view of ISH at 5-dpf showing expression of liver-specific markers sepp1b (D), cp (E), and fabp10a (F). L, liver, ib; intestinal bulb; gb, gas-bladder; y, yolk.
Figure 2. Disruption of cdipt expression and PtdIns synthesis in hi559 larvae
(A) The retroviral insertion (triangle) was mapped to the first intron of the cdipt gene. White boxes indicate exons, grey boxes introns. (B) cdipt expression is disurpted in hi559 larvae (RT-PCR amplification of three different regions, indicated in A). (C-E) Developmental expression pattern of cdipt in wild-type embryos. Note the absence of cdipt expression in hi559 larvae (wild-type, F, left; mutant, F, right). Intestine (arrow), liver (arrowhead). (F) PtdIns synthesis is disrupted in hi559 larvae. The bar chart represents values from three biological replicates.
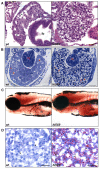
Figure 3. Histopathological abnormalities of hi559 liver
(A) H&E-stained sagittal sections of 5-dpf wild-type and hi559 liver. Mutant liver architecture is abnormal with vacuolar appearance and smaller sinuses (asterisk). (B) Toluedene staining of semi-thin transverse sections reveals large, vacuolated hepatocytes with marginalized nuclei (arrow) in hi559 liver. (C) Whole mount ORO staining of 5-dpf larvae shows fatty liver (dotted line) in the mutant. (D) ORO staining of frozen tissue sections of 5-dpf larvae reveals substantial steatosis in hi559 hepatocytes. Arrows indicate lipid droplets (red), arrowheads nuclei. ib, intestinal bulb; in, intestine; L, liver; y, yolk; wt, wild-type. Scale bars: A, 20 μM; B, 50 μM; D, 20 μm.
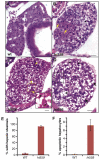
Figure 4. NAFLD progression in hi559 liver
H&E sections of wild-type liver at 6-dpf (A), and stages of NAFLD progression in hi559 liver at 5-dpf (B), 5.5-dpf (C), and 6-dpf (D). At early stage of NAFLD at 5-dpf, foci of microvesicular (arrow) and macrovesicular (arrowhead) steatosis are evident with no apparent apoptosis. At 5.5-dpf, most of the hepatocytes exhibit macrovesicular steatosis with a few apoptotic hepatocytes (arrow, the dotted line area magnified in the inset). At 6-dpf, hepatocellular ballooning (arrow), often with perinuclear hyaline inclusions (arrowhead), and necrotic foci (dotted line) are apparent. (E) Bar-charts showing percentages of embryos with hepatic steatosis at 5-dpf. (F) Bar-charts showing percentages of apoptotic hepatocytes in 6-dpf liver. Data are representative of 5 biological replicates. Scale bars: 20 μM.
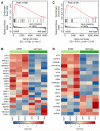
Figure 5. Deregulation of genes involved in ERSR
GSEA enrichment plots and expression profile (shown by heat-map) of genes involved in the ERSR/UPR pathway (A-B) and integrated stress-response pathway (C-D). Running enrichment score (ES, top) and signal-to-noise ratio (bottom) used for ranking genes (positive: upregulated in mutant; negative: downregulated in mutant) are shown in the GSEA plots. Vertical bars (middle) indicate the position of genes in the ERSR/UPR within the sorted microarray data, showing enrichment among genes upregulated in hi559. Normalized ES (NES) and nominal _p_-value, below.
Figure 6. Preferential upregulation of ERSR/UPR genes in the liver of hi559 embryos
ISH of ERSR markers in wild-type (top) and hi559 embryos (bottom) at 5-dpf. The respective gene symbols are indicated in each panel. Note the elevated expression in the hi559 liver (arrow).
Figure 7. Ultrastructural pathology of hepatocytes
(A-D) Electron-micrographic comparison of wild-type and hi559 hepatocytes. Cytoplasmic clearing (asterisk, B), ER luminal swelling (triangle, D), and abnormal mitochondria are frequently observed in hi559 hepatocytes. (E) hi559 hepatocyte shows mitochondrial damage and excessive ER luminal vacuolization. The ER lumens are filled with granular materials. (F) The hi559 hepatocytes often contain membrane-bound lipids (lipolysosomes, arrow) of variable electron density. (G) Autophagosome-like structures (arrow) containing reticular materials are noticed within hi559 hepatocytes. (H) Presence of a macrophage (arrow) adjacent to necrotic hepatocytes. mc, mitochondria; n, nucleus; er: endoplasmic reticulum. wt, wild-type; mut, hi559 mutant. Scale bars: A-B & F, 2 μm_; C-E_ & G,-H, 500 nm.
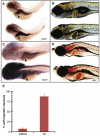
Figure 8. Tunicamycin-induced ER stress causes fatty liver in wild-type larvae
(A) Elevated expression of hspa5 in hi559 liver at 4-dpf by ISH (arrow). (B) Chronic exposure of 1 μM tunicamycin from 3.5-dpf to 5.5-dpf causes globular darkish liver (yellow outline) similar to hi559. (C) hspa5 expression is ubiquitously elevated, including liver (arrow) in tunicamycin-treated larvae. (D) ORO staining shows presence of fatty liver (yellow outline) in tunicamycin-treated larvae. (E) Bar-charts indicating percentage of larvae with hepatic steatosis at 5.5-dpf in DMSO and tunicamycin (TM) treated groups. Data are representative of 3 clutches (n=55).
Similar articles
- Dysregulated phosphatidylinositol signaling promotes endoplasmic-reticulum-stress-mediated intestinal mucosal injury and inflammation in zebrafish.
Thakur PC, Davison JM, Stuckenholz C, Lu L, Bahary N. Thakur PC, et al. Dis Model Mech. 2014 Jan;7(1):93-106. doi: 10.1242/dmm.012864. Epub 2013 Oct 17. Dis Model Mech. 2014. PMID: 24135483 Free PMC article. - Phosphatidylinositol synthase is required for lens structural integrity and photoreceptor cell survival in the zebrafish eye.
Murphy TR, Vihtelic TS, Ile KE, Watson CT, Willer GB, Gregg RG, Bankaitis VA, Hyde DR. Murphy TR, et al. Exp Eye Res. 2011 Oct;93(4):460-74. doi: 10.1016/j.exer.2011.06.010. Epub 2011 Jun 23. Exp Eye Res. 2011. PMID: 21722635 Free PMC article. - Activating transcription factor 6 plays protective and pathological roles in steatosis due to endoplasmic reticulum stress in zebrafish.
Cinaroglu A, Gao C, Imrie D, Sadler KC. Cinaroglu A, et al. Hepatology. 2011 Aug;54(2):495-508. doi: 10.1002/hep.24396. Epub 2011 Jun 23. Hepatology. 2011. PMID: 21538441 Free PMC article. - Nutritional related liver disease: targeting the endoplasmic reticulum stress.
Kammoun HL, Hainault I, Ferré P, Foufelle F. Kammoun HL, et al. Curr Opin Clin Nutr Metab Care. 2009 Nov;12(6):575-82. doi: 10.1097/MCO.0b013e32833189db. Curr Opin Clin Nutr Metab Care. 2009. PMID: 19726979 Review. - Endoplasmic reticulum proteostasis in hepatic steatosis.
Baiceanu A, Mesdom P, Lagouge M, Foufelle F. Baiceanu A, et al. Nat Rev Endocrinol. 2016 Dec;12(12):710-722. doi: 10.1038/nrendo.2016.124. Epub 2016 Aug 12. Nat Rev Endocrinol. 2016. PMID: 27516341 Review.
Cited by
- Zebrafish models of human liver development and disease.
Wilkins BJ, Pack M. Wilkins BJ, et al. Compr Physiol. 2013 Jul;3(3):1213-30. doi: 10.1002/cphy.c120021. Compr Physiol. 2013. PMID: 23897685 Free PMC article. Review. - The emerging use of zebrafish to model metabolic disease.
Seth A, Stemple DL, Barroso I. Seth A, et al. Dis Model Mech. 2013 Sep;6(5):1080-8. doi: 10.1242/dmm.011346. Dis Model Mech. 2013. PMID: 24046387 Free PMC article. Review. - Homeostatic generation of reactive oxygen species protects the zebrafish liver from steatosis.
Nussbaum JM, Liu LJ, Hasan SA, Schaub M, McClendon A, Stainier DY, Sakaguchi TF. Nussbaum JM, et al. Hepatology. 2013 Oct;58(4):1326-38. doi: 10.1002/hep.26551. Epub 2013 Aug 14. Hepatology. 2013. PMID: 23744565 Free PMC article. - Metabolic Biomarkers In Midtrimester Maternal Plasma Can Accurately Predict Adverse Pregnancy Outcome in Patients with SLE.
Lee SM, Lee EM, Park JK, Jeon HS, Oh S, Hong S, Jung YM, Kim BJ, Kim SM, Norwitz ER, Lee EB, Louangsenlath S, Park CW, Jun JK, Park JS, Lee DY. Lee SM, et al. Sci Rep. 2019 Oct 23;9(1):15169. doi: 10.1038/s41598-019-51285-8. Sci Rep. 2019. PMID: 31645572 Free PMC article. - Molecularly defined unfolded protein response subclasses have distinct correlations with fatty liver disease in zebrafish.
Vacaru AM, Di Narzo AF, Howarth DL, Tsedensodnom O, Imrie D, Cinaroglu A, Amin S, Hao K, Sadler KC. Vacaru AM, et al. Dis Model Mech. 2014 Jul;7(7):823-35. doi: 10.1242/dmm.014472. Dis Model Mech. 2014. PMID: 24973751 Free PMC article.
References
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. - PubMed
- Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 7:195–203. - PubMed
- Stuckenholz C, Ulanch PE, Bahary N. From guts to brains: using zebrafish genetics to understand the innards of organogenesis. Curr Top Dev Biol. 2005;65:47–82. - PubMed
- Wallace KN, Pack M. Unique and conserved aspects of gut development in zebrafish. Dev Biol. 2003;255:12–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA047904/CA/NCI NIH HHS/United States
- P30CA047904/CA/NCI NIH HHS/United States
- R21DK073177/DK/NIDDK NIH HHS/United States
- R21 DK073177/DK/NIDDK NIH HHS/United States
- R01 HD050872/HD/NICHD NIH HHS/United States
- R01 AA018886/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous