Evolution of olfaction in non-avian theropod dinosaurs and birds - PubMed (original) (raw)
Comparative Study
. 2011 Dec 22;278(1725):3625-34.
doi: 10.1098/rspb.2011.0238. Epub 2011 Apr 13.
Affiliations
- PMID: 21490022
- PMCID: PMC3203493
- DOI: 10.1098/rspb.2011.0238
Comparative Study
Evolution of olfaction in non-avian theropod dinosaurs and birds
Darla K Zelenitsky et al. Proc Biol Sci. 2011.
Abstract
Little is known about the olfactory capabilities of extinct basal (non-neornithine) birds or the evolutionary changes in olfaction that occurred from non-avian theropods through modern birds. Although modern birds are known to have diverse olfactory capabilities, olfaction is generally considered to have declined during avian evolution as visual and vestibular sensory enhancements occurred in association with flight. To test the hypothesis that olfaction diminished through avian evolution, we assessed relative olfactory bulb size, here used as a neuroanatomical proxy for olfactory capabilities, in 157 species of non-avian theropods, fossil birds and living birds. We show that relative olfactory bulb size increased during non-avian maniraptoriform evolution, remained stable across the non-avian theropod/bird transition, and increased during basal bird and early neornithine evolution. From early neornithines through a major part of neornithine evolution, the relative size of the olfactory bulbs remained stable before decreasing in derived neoavian clades. Our results show that, rather than decreasing, the importance of olfaction actually increased during early bird evolution, representing a previously unrecognized sensory enhancement. The relatively larger olfactory bulbs of earliest neornithines, compared with those of basal birds, may have endowed neornithines with improved olfaction for more effective foraging or navigation skills, which in turn may have been a factor allowing them to survive the end-Cretaceous mass extinction.
Figures
Figure 1.
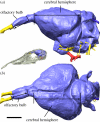
Virtual brain endocast of Lithornis plebius, (a) in left lateral and (b) dorsal view, showing the location of the olfactory bulbs and cerebral hemispheres. The greatest linear dimension of the olfactory bulb and cerebral hemisphere, regardless of orientation, was used to calculate olfactory ratios (only rostrocaudal dimensions illustrated). Red features represent blood vessels, yellow features represent cranial nerves. Scale bar, 5 mm. Inset (not to scale) shows the position of the brain endocast within the skull of Lithornis promiscuus.
Figure 2.
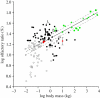
Plot of log-transformed olfactory ratio versus log-transformed body mass in avian and non-avian theropods. No significant correlation is observed between olfactory ratio and body mass among birds when phylogeny is considered (_r_2 = 0.009, p = 0.26). In contrast, a significant positive correlation is observed between olfactory ratio and body mass among non-avian theropods (_r_2 = 0.8, p < 1.27e − 7), indicating that olfactory ratios increase with body mass among non-avian theropods. The non-avian theropod regression (solid black line) and its extrapolation (black dashed line) bisect the distribution of olfactory ratios for birds. The majority of neornithine species basal to the common ancestor of Charadriiformes and Passeriformes have higher olfactory ratios than more derived taxa. Most basal birds fall near the non-avian theropod regression. The fossil diving bird Hesperornis plots near the extant divers Gavia immer (loon, G) and Pygoscelis adeliae (Adelie penguin, P). The error associated with Hesperornis reflects the uncertainty of its cerebral hemisphere length (see §3). The dromaeosaurid Bambiraptor (B) plots near Cathartes aura (turkey vulture, open circle) and Phoebastria nigripes (black-footed albatross, solid circle). The extinct palaeognath Lithornis (L) has high olfactory ratios. Green diamonds, dromaeosaurids; green squares, tyrannosaurids; green triangles, allosauroids; green stars, ceratosaurs; green circles, ornithomimosaurs; inverted green triangle, Citipati; green crosses, Dilong and Troodon; red circle, Archaeopteryx; red triangle, Confuciusornis; red cross, Ichthyornis; red diamond, Hesperornis; black circles, neornithines basal to charadriiform–passeriform common ancestor (based on molecular phylogeny); white circles, neornithines more derived than charadriiform–passeriform common ancestor (based on molecular phylogeny). Dashed grey lines represent the 95% confidence interval of the non-avian theropod regression.
Figure 3.
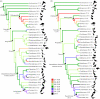
Higher order phylogeny of Aves showing maximum-parsimony ancestral state reconstruction of olfactory ratios. (a) Molecular phylogeny based primarily on Hackett et al. [64]. (b) Morphological phylogeny based on Livezey & Zusi [65]. Major increases or decreases in olfactory ratio ancestral states are denoted with (+) and (−), respectively. Through avian evolution, major increases in olfactory ratios have occurred independently in many different lineages, primarily in clades basal to the common ancestor of Charadriiformes and Passeriformes. Significant decreases in olfactory ratios are prevalent in clades more derived than this common ancestor. Numbers between parentheses represent mean olfactory ratios for clades. See the electronic supplementary material for details.
Figure 4.
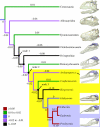
Phylogeny of non-avian theropods and early birds showing maximum-parsimony ancestral state reconstruction of olfactory ratio residuals relative to the non-avian theropod regression. Residuals increase from the Maniraptoriformes common ancestor (node 1) to the Eumaniraptora common ancestor (node 2). Residuals then remain constant across the non-avian theropod/bird transition, between the Eumaniraptora common ancestor (node 2) and the Pygostylia common ancestor (node 3). Within Aves, residuals increase from negative values in basal birds to strongly positive values in neornithines, indicating that olfactory ratios increase and surpass values predicted by the regression for non-avian theropods. These results reveal that olfactory capabilities improved during the evolution from non-avian theropods to modern birds. Yellow box, Aves; blue box, Neornithes. Skulls with endocasts are, from top to bottom, Majungasaurus crenatissimus, Allosaurus fragilis, Tyrannosaurus rex, Struthiomimus altus, Bambiraptor feinbergi, Archaeopteryx lithographica, Ichthyornis dispar, Lithornis sp. and Presbyornis sp. Skulls are not to scale.
Similar articles
- Olfactory acuity in theropods: palaeobiological and evolutionary implications.
Zelenitsky DK, Therrien F, Kobayashi Y. Zelenitsky DK, et al. Proc Biol Sci. 2009 Feb 22;276(1657):667-73. doi: 10.1098/rspb.2008.1075. Proc Biol Sci. 2009. PMID: 18957367 Free PMC article. - Olfactory receptor repertoire size in dinosaurs.
Hughes GM, Finarelli JA. Hughes GM, et al. Proc Biol Sci. 2019 Jun 12;286(1904):20190909. doi: 10.1098/rspb.2019.0909. Epub 2019 Jun 12. Proc Biol Sci. 2019. PMID: 31185870 Free PMC article. - Air-filled postcranial bones in theropod dinosaurs: physiological implications and the 'reptile'-bird transition.
Benson RB, Butler RJ, Carrano MT, O'Connor PM. Benson RB, et al. Biol Rev Camb Philos Soc. 2012 Feb;87(1):168-93. doi: 10.1111/j.1469-185X.2011.00190.x. Epub 2011 Jul 7. Biol Rev Camb Philos Soc. 2012. PMID: 21733078 Review. - Ultramicrostructural reductions in teeth: implications for dietary transition from non-avian dinosaurs to birds.
Li Z, Wang CC, Wang M, Chiang CC, Wang Y, Zheng X, Huang EW, Hsiao K, Zhou Z. Li Z, et al. BMC Evol Biol. 2020 Apr 21;20(1):46. doi: 10.1186/s12862-020-01611-w. BMC Evol Biol. 2020. PMID: 32316913 Free PMC article. - New Perspectives on the Ontogeny and Evolution of Avian Locomotion.
Heers AM. Heers AM. Integr Comp Biol. 2016 Sep;56(3):428-41. doi: 10.1093/icb/icw065. Epub 2016 Jul 1. Integr Comp Biol. 2016. PMID: 27371381 Review.
Cited by
- From sauropsids to mammals and back: New approaches to comparative cortical development.
Montiel JF, Vasistha NA, Garcia-Moreno F, Molnár Z. Montiel JF, et al. J Comp Neurol. 2016 Feb 15;524(3):630-45. doi: 10.1002/cne.23871. Epub 2015 Aug 20. J Comp Neurol. 2016. PMID: 26234252 Free PMC article. Review. - Olfactory discrimination ability of South African fur seals (Arctocephalus pusillus) for enantiomers.
Kim S, Amundin M, Laska M. Kim S, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013 Jun;199(6):535-44. doi: 10.1007/s00359-012-0759-5. Epub 2012 Sep 26. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013. PMID: 23011284 - Penguins reduced olfactory receptor genes common to other waterbirds.
Lu Q, Wang K, Lei F, Yu D, Zhao H. Lu Q, et al. Sci Rep. 2016 Aug 16;6:31671. doi: 10.1038/srep31671. Sci Rep. 2016. PMID: 27527385 Free PMC article. - Diversity in olfactory bulb size in birds reflects allometry, ecology, and phylogeny.
Corfield JR, Price K, Iwaniuk AN, Gutierrez-Ibañez C, Birkhead T, Wylie DR. Corfield JR, et al. Front Neuroanat. 2015 Jul 29;9:102. doi: 10.3389/fnana.2015.00102. eCollection 2015. Front Neuroanat. 2015. PMID: 26283931 Free PMC article. - A reappraisal of Cerebavis cenomanica (Aves, Ornithurae), from Melovatka, Russia.
Walsh SA, Milner AC, Bourdon E. Walsh SA, et al. J Anat. 2016 Aug;229(2):215-27. doi: 10.1111/joa.12406. Epub 2015 Nov 10. J Anat. 2016. PMID: 26553244 Free PMC article.
References
- Britt B. B., Makovicky P. J., Gauthier J., Bond N. 1998. Postcranial pneumatization in Archaeopteryx. Nature 395, 374–37610.1038/26469 (doi:10.1038/26469) - DOI - DOI
- Currie P. J. 1987. Bird-like characteristics of the jaws and teeth of troodontid theropods (Dinosauria, Saurischia). J. Vertebr. Paleontol. 7, 72–8110.1080/02724634.1987.10011638 (doi:10.1080/02724634.1987.10011638) - DOI - DOI
- Erickson G. M., Rauhut O. W. M., Zhou Z., Turner A. H., Inouye B. D., Hu D., Norell M. A. 2009. Was dinosaurian physiology inherited by birds? Reconciling slow growth in Archaeopteryx. PLoS ONE 4, e7390.10.1371/journal.pone.0007390 (doi:10.1371/journal.pone.0007390) - DOI - DOI - PMC - PubMed
- Ji Q., Currie P. J., Norell M. A., Ji A. 1998. Two feathered dinosaurs from northeastern China. Nature 393, 753–76110.1038/31635 (doi:10.1038/31635) - DOI - DOI
- Kurochkin E. N., Dyke G. J., Saveliev S. V., Pervushov E. M., Popov E. V. 2007. A fossil brain from the Cretaceous of European Russia and avian sensory evolution. Biol. Lett. 3, 309–31310.1098/rsbl.2006.0617 (doi:10.1098/rsbl.2006.0617) - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources