Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults - PubMed (original) (raw)
Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults
B C Dickerson et al. Neurology. 2011.
Abstract
Objective: Since Alzheimer disease (AD) neuropathology is thought to develop years before dementia, it may be possible to detect subtle AD-related atrophy in preclinical AD. Here we hypothesized that the "disease signature" of AD-related cortical thinning, previously identified in patients with mild AD dementia, would be useful as a biomarker to detect anatomic abnormalities consistent with AD in cognitively normal (CN) adults who develop AD dementia after longitudinal follow-up.
Methods: We studied 2 independent samples of adults who were CN when scanned. In sample 1, 8 individuals developing AD dementia (CN-AD converters) after an average of 11.1 years were compared to 25 individuals who remained CN (CN-stable). In sample 2, 7 CN-AD converters (average follow-up 7.1 years) were compared to 25 CN-stable individuals.
Results: AD-signature cortical thinning in CN-AD converters in both samples was remarkably similar, about 0.2 mm (p < 0.05). Despite this small absolute difference, Cohen d effect sizes for these differences were very large (> 1). Of the 11 CN individuals with baseline low AD-signature thickness (≥ 1 SD below cohort mean), 55% developed AD dementia over nearly the next decade, while none of the 9 high AD-signature thickness individuals (≥ 1 SD above mean) developed dementia. This marker predicted time to diagnosis of dementia (hazard ratio = 3.4, p < 0.0005); 1 SD of thinning increased dementia risk by 3.4.
Conclusions: By focusing on cortical regions known to be affected in AD dementia, subtle but reliable atrophy is identifiable in asymptomatic individuals nearly a decade before dementia, making this measure a potentially important imaging biomarker of early neurodegeneration.
Figures
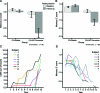
Figure 1. Baseline and longitudinal clinical characteristics of the 2 samples
(A, B) Baseline and follow-up episodic memory Z scores from the 2 samples, illustrating the stability (or even slight improvement in sample 2) of performance in the cognitively normal (CN)-stable groups over nearly a decade of follow-up. In contrast, the CN-Alzheimer disease (AD) converters performed normally at baseline but declined substantially over nearly a decade of follow-up by the time of diagnosis of AD dementia. (C, D) Detailed illustration of cognitive decline in each of the CN-AD converter individuals. (C) Annual Clinical Dementia Rating (CDR)–sum of boxes scores increased from normal (0) at baseline to mild dementia in each of the 8 individuals in the Massachusetts General Hospital sample. (D) Annual episodic memory Z scores declined from normal to substantially impaired in each of the 7 individuals in the Rush sample. Breaks in lines indicate years for which data were not available.
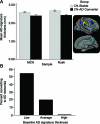
Figure 2. Thinner cortex in regions associated with Alzheimer disease (AD) in asymptomatic adults who eventually develop dementia
(A) For each of the 2 samples, the average cortical thickness of the AD-signature regions was slightly thinner in the cognitively normal (CN)-AD converter group than the CN-stable group at the time of the baseline MRI scan. The absolute difference in the means of the groups for each sample is less than ¼ mm, but because variance is small the Cohen d effect size between the groups is very large for each sample (>1.2). Inset illustrates AD-signature regions of interest. (B) Illustration of the risk of AD dementia as a function of AD-signature thickness from baseline MRI scans in the pooled group of all participants in this study. For the group of 9 individuals with high AD-signature thickness, none developed AD dementia in the follow-up period, while for the group of 45 individuals with average AD-signature thickness, 20% developed AD dementia in the follow-up period. A striking 55% of the 11 individuals with low AD-signature thickness developed AD dementia in the follow-up period.
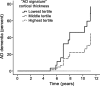
Figure 3. Alzheimer disease (AD)-signature MRI biomarker predicts time to dementia in people who were cognitively normal when scanned
Univariate survival plot of predicted time to AD dementia for hypothetical average study participants with AD-signature cortical thickness in the lowest (smallest) tertile, middle tertile, and highest tertile. The displayed survival curves are therefore model predictions and do not directly represent subject results. AD-signature thickness was predictive of progression from normal cognition to AD dementia (hazard ratio 3.5 for 1 SD decrease in thickness; 95% confidence interval 2.0–6.4; p < 0.00005) in the model. The mean thickness of the lowest tertile was 1.1 standard deviations thinner than the mean of the entire group of all participants, the mean of the middle tertile was at approximately the mean of the entire group, and the mean of the highest tertile was 1.1 standard deviations thicker than the mean.
Similar articles
- The cortical signature of prodromal AD: regional thinning predicts mild AD dementia.
Bakkour A, Morris JC, Dickerson BC. Bakkour A, et al. Neurology. 2009 Mar 24;72(12):1048-55. doi: 10.1212/01.wnl.0000340981.97664.2f. Epub 2008 Dec 24. Neurology. 2009. PMID: 19109536 Free PMC article. - MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults.
Dickerson BC, Wolk DA; Alzheimer's Disease Neuroimaging Initiative. Dickerson BC, et al. Neurology. 2012 Jan 10;78(2):84-90. doi: 10.1212/WNL.0b013e31823efc6c. Epub 2011 Dec 21. Neurology. 2012. PMID: 22189451 Free PMC article. - Spatially distinct atrophy is linked to β-amyloid and tau in preclinical Alzheimer disease.
Wang L, Benzinger TL, Hassenstab J, Blazey T, Owen C, Liu J, Fagan AM, Morris JC, Ances BM. Wang L, et al. Neurology. 2015 Mar 24;84(12):1254-60. doi: 10.1212/WNL.0000000000001401. Epub 2015 Feb 25. Neurology. 2015. PMID: 25716355 Free PMC article. - Relevance of magnetic resonance imaging for early detection and diagnosis of Alzheimer disease.
Teipel SJ, Grothe M, Lista S, Toschi N, Garaci FG, Hampel H. Teipel SJ, et al. Med Clin North Am. 2013 May;97(3):399-424. doi: 10.1016/j.mcna.2012.12.013. Epub 2013 Feb 1. Med Clin North Am. 2013. PMID: 23642578 Review. - Preclinical Alzheimer disease: identification of cases at risk among cognitively intact older individuals.
Lazarczyk MJ, Hof PR, Bouras C, Giannakopoulos P. Lazarczyk MJ, et al. BMC Med. 2012 Oct 25;10:127. doi: 10.1186/1741-7015-10-127. BMC Med. 2012. PMID: 23098093 Free PMC article. Review.
Cited by
- Life-course blood pressure in relation to brain volumes.
Power MC, Schneider AL, Wruck L, Griswold M, Coker LH, Alonso A, Jack CR Jr, Knopman D, Mosley TH, Gottesman RF. Power MC, et al. Alzheimers Dement. 2016 Aug;12(8):890-9. doi: 10.1016/j.jalz.2016.03.012. Epub 2016 Apr 29. Alzheimers Dement. 2016. PMID: 27139841 Free PMC article. - Association between surgical admissions, cognition, and neurodegeneration in older people: a population-based study from the UK Biobank.
Taylor J, Robledo KP, Medel V, Heller G, Payne T, Wehrman J, Casey C, Yang PF, Krause BM, Lennertz R, Naismith S, Teixeira-Pinto A, Sanders RD. Taylor J, et al. Lancet Healthy Longev. 2024 Sep;5(9):100623. doi: 10.1016/j.lanhl.2024.07.006. Epub 2024 Sep 5. Lancet Healthy Longev. 2024. PMID: 39245058 Free PMC article. - Masticatory Function, Sex, and Risk of Dementia Among Older Adults: A Population-Based Cohort Study.
Oh DJ, Han JW, Kim JS, Kim TH, Kwak KP, Kim BJ, Kim SG, Kim JL, Moon SW, Park JH, Ryu SH, Youn JC, Lee DY, Lee DW, Lee SB, Lee JJ, Jhoo JH, Kim KW. Oh DJ, et al. J Korean Med Sci. 2024 Sep 23;39(36):e246. doi: 10.3346/jkms.2024.39.e246. J Korean Med Sci. 2024. PMID: 39315441 Free PMC article. - Retinal imaging with hand-held optical coherence tomography in older people with or without postoperative delirium after hip fracture surgery: A feasibility study.
Noah AM, Spendlove J, Tu Z, Proudlock F, Constantinescu CS, Gottlob I, Auer DP, Dineen RA, Moppett IK. Noah AM, et al. PLoS One. 2024 Jul 16;19(7):e0305964. doi: 10.1371/journal.pone.0305964. eCollection 2024. PLoS One. 2024. PMID: 39012893 Free PMC article. - Shapes of the trajectories of 5 major biomarkers of Alzheimer disease.
Jack CR Jr, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Lowe V, Kantarci K, Bernstein MA, Senjem ML, Gunter JL, Boeve BF, Trojanowski JQ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Knopman DS; Alzheimer’s Disease Neuroimaging Initiative. Jack CR Jr, et al. Arch Neurol. 2012 Jul;69(7):856-67. doi: 10.1001/archneurol.2011.3405. Arch Neurol. 2012. PMID: 22409939 Free PMC article.
References
- Amieva H, Le Goff M, Millet X, et al. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol 2008;64:492–498 - PubMed
- Rubin EH, Storandt M, Miller JP, et al. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Arch Neurol 1998;55:395–401 - PubMed
- Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer's disease. Stat Med 2000;19:1555–1566 - PubMed
- Howieson DB, Dame A, Camicioli R, Sexton G, Payami H, Kaye JA. Cognitive markers preceding Alzheimer's dementia in the healthy oldest old. J Am Geriatr Soc 1997;45:584–589 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50-AG005134/AG/NIA NIH HHS/United States
- P41-RR14075/RR/NCRR NIH HHS/United States
- P01-AG14449/AG/NIA NIH HHS/United States
- R01-AG29411/AG/NIA NIH HHS/United States
- R01-AG17917/AG/NIA NIH HHS/United States
- R21-AG29840/AG/NIA NIH HHS/United States
- R01-AG10688/AG/NIA NIH HHS/United States
- U24-RR021382/RR/NCRR NIH HHS/United States
- R01 AG029411/AG/NIA NIH HHS/United States
- P30-AG10161/AG/NIA NIH HHS/United States
- P01-AG09466/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical