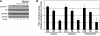Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice - PubMed (original) (raw)
. 2011 May;121(5):1858-70.
doi: 10.1172/JCI43378. Epub 2011 Apr 1.
Benjamin T Bikman, Li-Ping Wang, Guan Yuguang, Katherine M Sargent, Sarada Bulchand, Trina A Knotts, Guanghou Shui, Deborah J Clegg, Markus R Wenk, Michael J Pagliassotti, Philipp E Scherer, Scott A Summers
Affiliations
- PMID: 21490391
- PMCID: PMC3083776
- DOI: 10.1172/JCI43378
Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice
William L Holland et al. J Clin Invest. 2011 May.
Abstract
Obesity is associated with an enhanced inflammatory response that exacerbates insulin resistance and contributes to diabetes, atherosclerosis, and cardiovascular disease. One mechanism accounting for the increased inflammation associated with obesity is activation of the innate immune signaling pathway triggered by TLR4 recognition of saturated fatty acids, an event that is essential for lipid-induced insulin resistance. Using in vitro and in vivo systems to model lipid induction of TLR4-dependent inflammatory events in rodents, we show here that TLR4 is an upstream signaling component required for saturated fatty acid-induced ceramide biosynthesis. This increase in ceramide production was associated with the upregulation of genes driving ceramide biosynthesis, an event dependent of the activity of the proinflammatory kinase IKKβ. Importantly, increased ceramide production was not required for TLR4-dependent induction of inflammatory cytokines, but it was essential for TLR4-dependent insulin resistance. These findings suggest that sphingolipids such as ceramide might be key components of the signaling networks that link lipid-induced inflammatory pathways to the antagonism of insulin action that contributes to diabetes.
Figures
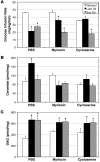
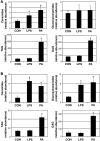
Figure 1. Lard oil infusion inhibits insulin-stimulated glucose uptake in a ceramide-dependent manner.
(A) Whole body glucose disposal was assessed by hyperinsulin-euglycemic clamp during infusion with lard oil (black bars), soy oil (white bars), or glycerol (gray bars) following treatment with inhibitors myriocin, cycloserine, or PBS. (B and C) Ceramide (B) and DAG (C) content was enzymatically determined using the DAG-kinase assay from soleus muscle following 6 hours of lipid infusion. Values are expressed as mean ± SEM (n = 8). *P < 0.05 for lard or soy oil versus glycerol within a given treatment.
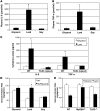
Figure 2. Lard oil infusion increases circulating inflammatory cytokine concentrations and impairs insulin-stimulated glucose disposal in a TLR4-dependent manner.
(A and B) Plasma concentrations of IL-6 (A) and TNF-α (B) were determined using ELISA following a 6-hour infusion of glycerol, lard oil, or soy oil emulsions into male Sprague-Dawley rats. (C) Serum concentrations of IL-6 and TNF-α were measured using ELISA after 6 hours of glycerol or lard oil infusion into WT mice or TLR4-defective mice. (D) Whole body glucose disposal was assessed by hyperinsulinemic-euglycemic clamp initiated after 4.5 hours of glycerol, lard oil, or soy oil infusion into WT mice (white bars) or TLR4-defective mice (black bars). (E) Whole body glucose disposal was assessed by hyperinsulinemic-euglycemic clamp initiated after 4.5 hours of glycerol (white bars) or lard oil (black bars) infusion into WT mice, MyD88-null mice, or TLR2-null mice. Values are expressed as mean ± SEM (n = 6). *P < 0.05 for treatment versus glycerol control.
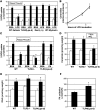
Figure 3. LPS and palmitate (PA) impair insulin-stimulated glucose uptake in isolated muscles via sphingolipid- and TLR4-dependent mechanisms.
(A and B) Bisected soleus muscle strips from male Sprague Dawley rats were incubated for 6 hours in the presence or absence of LPS (100 ng/ml). (A) 2-DOG uptake was quantified under basal (white) or insulin-stimulated (black; 300 μU/ml) conditions following treatment with LPS or BSA control for 6 hours. *P < 0.05 compared with BSA conditions. (B) Ceramide was enzymatically quantified after 0, 4, or 6 hours of LPS treatment. *P < 0.05 for PA at 4 and 6 hours versus 0 hours. (C) Whole soleus muscles from WT, TLR2-null, or TLR4-defective mice were incubated for 6 hours in PA (1 mM) prior to measuring hexose uptake under basal (white bars) or insulin-stimulated (300 μU/ml; black bars) conditions. *P < 0.05 compared with BSA conditions. (D and E) After duplicate 6-hour treatments, ceramide (D) and DAG (E) were enzymatically quantified from lipid extracts of whole soleus muscle (n = 6) following treatment with BSA (white bars) and PA (black bars). *P < 0.05 for PA versus BSA. (F) The rate of PA oxidation was quantified ex vivo from whole soleus muscle of WT and TLR4-defective mice. *P < 0.05 for Tlr4lps-d compared with WT. Values are expressed as mean ± SEM (n = 6–8).
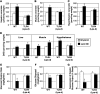
Figure 4. TLR4 signaling is essential for lipid-induced ceramide accumulation.
Following 6 hours of intravenous infusion of glycerol (white bars) or lard oil (black bars), ceramides (A–C) were enzymatically determined from liver (A), muscle (B), or hypothalamus (C). (D) DAG was simultaneously measured in the same tissues. (E–G) Glucose kinetics were determined during hyperinsulinemic-euglycemic clamps (4 mU/kg/min insulin). (E) The glucose infusion rate required to maintain euglycemia was recorded. (F) Glucose disposal was calculated from 3H-glucose turnover. (G) Hepatic glucose production was calculated during basal and insulin-stimulated conditions and presented as percent suppression by insulin (n = 5–6). *P < 0.05 for lard oil compared with glycerol.
Figure 5. LPS and PA elicit an increase in sphingolipd synthesis.
Lipids were determined in murine C2C12 myotubes (A) and RAW264.7 macrophages (B) challenged with PA and LPS. LC-MS was performed to determine ceramides, glucosylceramides, TAG, and DAG in cells treated with LPS (1 μg/ml for myotubes; 100 ng/ml for macrophages) and PA (0.75 mM for myotubes; 0.45 mM for macrophages) for 16 hours. Values are expressed as mean ± SEM (n = 4). *P < 0.05 for treatment compared with BSA control (CON).
Figure 6. Ceramide is necessary for LPS and PA inhibition on insulin signaling at Akt/PKB in myotubes but not for LPS and PA induction of TNF-α in macrophages.
(A) C2C12 myotubes were treated with PA (0.75 mM) and LPS (1 μg/ml) for 8 hours in the presence or absence of myriocin (2 μg/ml), followed by 10 minutes of insulin stimulation (100 nM). (B) RAW264.7 macrophages were treated with LPS (100 ng/ml) or PA or linoleate (LA) (0.45 mM) in conjunction with either myriocin (10 μM) or fumonisin B1 (50 μM) (B). Values are expressed as mean ± SEM (n = 4–5). *P < 0.05 for treatment versus actin control.
Figure 7. Overexpression of a dominant-negative IKKβ prevents ceramide accrual and LPS- and PA-induced insulin resistance.
(A) To confirm the lack of IKKβ action in the IKK-KD cells, IκBα levels were measured in WT and KD myotubes following LPS (1 μg/ml) treatment for 1 hour. (B and C) IKK-KD cells failed to accrue ceramides (B), as determined by LC-MS, but did accumulate DAG to relatively normal degrees when treated with LPS (1 μg/ml) or PA (0.75 mM) for 16 hours (C). (D) IKK-KD cells were resistant to the detrimental effects on Akt/PKB signaling compared with WT cells with PA (0.75 mM) and LPS (1 μg/ml) treatment for 12 hours with or without 1 hour myriocin (10 μM) pretreatment. Murine C2C12 myotubes responded to LPS and PA by selectively upregulating de novo ceramide synthesis in control cells, but not IKK-KD cells. (E) Quantitative real-time PCR was performed to determine transcript levels of the enzymes involved in ceramide synthesis on cells treated with LPS (1 μg/ml) and PA (0.75 mM) for 4 hours. *P < 0.05 for treatment compared with control. Values are expressed as mean ± SEM (n = 3–5). *P < 0.05 for treatment versus BSA control.
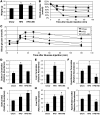
Figure 8. Suppression of IKKβ with NS restores insulin sensitivity via ceramide inhibition.
Male C57BL/6 mice were fed either a chow (10% of kcal from fat) or a high-fat diet (HFD; 60% of kcal from fat) from 5 weeks old. At 22 weeks, randomly assigned high-fat diet–fed mice were switched to a high-fat diet supplemented with 6 g/kg NS (HFD+NS) for 6 weeks. (A) The high-fat diet was associated with significant weight gain in both groups compared with chow-fed littermates. *P < 0.05 for HFD and HFD+NS compared with chow-fed mice. (B and C) Insulin and glucose tolerance were improved in the high-fat diet plus NS group. *P < 0.05 for HFD+NS compared with HFD alone. (D–I) Addition of NS to the high-fat diet effectively inhibited ceramide accrual in soleus, liver, and hypothalamus (D–F; *P < 0.05 for HFD compared with chow and HFD+NS) but exerted only an inhibitory effect on DAG in hypothalamus (I), not soleus or liver (G and H). Values are expressed as mean ± SEM (n = 8). *P < 0.05 for HFD compared with chow.
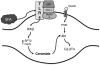
Figure 9. Diagram of possible events involved in TLR4 action on ceramides.
Similar articles
- Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity.
Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Kim F, et al. Circ Res. 2007 Jun 8;100(11):1589-96. doi: 10.1161/CIRCRESAHA.106.142851. Epub 2007 May 3. Circ Res. 2007. PMID: 17478729 - A high ratio of dietary n-3/n-6 polyunsaturated fatty acids improves obesity-linked inflammation and insulin resistance through suppressing activation of TLR4 in SD rats.
Liu HQ, Qiu Y, Mu Y, Zhang XJ, Liu L, Hou XH, Zhang L, Xu XN, Ji AL, Cao R, Yang RH, Wang F. Liu HQ, et al. Nutr Res. 2013 Oct;33(10):849-58. doi: 10.1016/j.nutres.2013.07.004. Epub 2013 Aug 9. Nutr Res. 2013. PMID: 24074743 - Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance.
Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Holland WL, et al. Cell Metab. 2007 Mar;5(3):167-79. doi: 10.1016/j.cmet.2007.01.002. Cell Metab. 2007. PMID: 17339025 - A global perspective on the crosstalk between saturated fatty acids and Toll-like receptor 4 in the etiology of inflammation and insulin resistance.
Li B, Leung JCK, Chan LYY, Yiu WH, Tang SCW. Li B, et al. Prog Lipid Res. 2020 Jan;77:101020. doi: 10.1016/j.plipres.2019.101020. Epub 2019 Dec 20. Prog Lipid Res. 2020. PMID: 31870728 Review. - Ceramides and mitochondrial fatty acid oxidation in obesity.
Fucho R, Casals N, Serra D, Herrero L. Fucho R, et al. FASEB J. 2017 Apr;31(4):1263-1272. doi: 10.1096/fj.201601156R. Epub 2016 Dec 21. FASEB J. 2017. PMID: 28003342 Review.
Cited by
- Inflammation and Insulin Resistance as Risk Factors and Potential Therapeutic Targets for Alzheimer's Disease.
Vinuesa A, Pomilio C, Gregosa A, Bentivegna M, Presa J, Bellotto M, Saravia F, Beauquis J. Vinuesa A, et al. Front Neurosci. 2021 Apr 23;15:653651. doi: 10.3389/fnins.2021.653651. eCollection 2021. Front Neurosci. 2021. PMID: 33967682 Free PMC article. Review. - Saturated and unsaturated fat induce hepatic insulin resistance independently of TLR-4 signaling and ceramide synthesis in vivo.
Galbo T, Perry RJ, Jurczak MJ, Camporez JP, Alves TC, Kahn M, Guigni BA, Serr J, Zhang D, Bhanot S, Samuel VT, Shulman GI. Galbo T, et al. Proc Natl Acad Sci U S A. 2013 Jul 30;110(31):12780-5. doi: 10.1073/pnas.1311176110. Epub 2013 Jul 9. Proc Natl Acad Sci U S A. 2013. PMID: 23840067 Free PMC article. - Linking inflammation to tumorigenesis in a mouse model of high-fat-diet-enhanced colon cancer.
Day SD, Enos RT, McClellan JL, Steiner JL, Velázquez KT, Murphy EA. Day SD, et al. Cytokine. 2013 Oct;64(1):454-62. doi: 10.1016/j.cyto.2013.04.031. Epub 2013 Jun 2. Cytokine. 2013. PMID: 23735174 Free PMC article. - CerS6-dependent ceramide synthesis in hypothalamic neurons promotes ER/mitochondrial stress and impairs glucose homeostasis in obese mice.
Hammerschmidt P, Steculorum SM, Bandet CL, Del Río-Martín A, Steuernagel L, Kohlhaas V, Feldmann M, Varela L, Majcher A, Quatorze Correia M, Klar RFU, Bauder CA, Kaya E, Porniece M, Biglari N, Sieben A, Horvath TL, Hornemann T, Brodesser S, Brüning JC. Hammerschmidt P, et al. Nat Commun. 2023 Nov 29;14(1):7824. doi: 10.1038/s41467-023-42595-7. Nat Commun. 2023. PMID: 38016943 Free PMC article. - Sphingolipid signaling in metabolic disorders.
Hla T, Dannenberg AJ. Hla T, et al. Cell Metab. 2012 Oct 3;16(4):420-34. doi: 10.1016/j.cmet.2012.06.017. Epub 2012 Sep 13. Cell Metab. 2012. PMID: 22982021 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- P01DK088761/DK/NIDDK NIH HHS/United States
- R01 DK081456/DK/NIDDK NIH HHS/United States
- TL1-DK081181/DK/NIDDK NIH HHS/United States
- R01DK081456-01/DK/NIDDK NIH HHS/United States
- R01 DK055758/DK/NIDDK NIH HHS/United States
- TL1 DK081181/DK/NIDDK NIH HHS/United States
- F32 DK083866/DK/NIDDK NIH HHS/United States
- P01 DK088761/DK/NIDDK NIH HHS/United States
- F32-DK083866/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases