Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice - PubMed (original) (raw)
. 2011 May;121(5):1709-21.
doi: 10.1172/JCI43792. Epub 2011 Apr 1.
Esther Hermano, Eyal Zcharia, Dina Rodkin, Raanan Bulvik, Victoria Doviner, Ariel M Rubinstein, Rivka Ishai-Michaeli, Ruth Atzmon, Yoav Sherman, Amichay Meirovitz, Tamar Peretz, Israel Vlodavsky, Michael Elkin
Affiliations
- PMID: 21490396
- PMCID: PMC3083784
- DOI: 10.1172/JCI43792
Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice
Immanuel Lerner et al. J Clin Invest. 2011 May.
Abstract
Ulcerative colitis (UC) is a chronic inflammatory bowel disease that is closely associated with colon cancer. Expression of the enzyme heparanase is clearly linked to colon carcinoma progression, but its role in UC is unknown. Here we demonstrate for what we believe to be the first time the importance of heparanase in sustaining the immune-epithelial crosstalk underlying colitis-associated tumorigenesis. Using histological specimens from UC patients and a mouse model of dextran sodium sulfate-induced colitis, we found that heparanase was constantly overexpressed and activated throughout the disease. We demonstrate, using heparanase-overexpressing transgenic mice, that heparanase overexpression markedly increased the incidence and severity of colitis-associated colonic tumors. We found that highly coordinated interactions between the epithelial compartment (contributing heparanase) and mucosal macrophages preserved chronic inflammatory conditions and created a tumor-promoting microenvironment characterized by enhanced NF-κB signaling and induction of STAT3. Our results indicate that heparanase generates a vicious cycle that powers colitis and the associated tumorigenesis: heparanase, acting synergistically with the intestinal flora, stimulates macrophage activation, while macrophages induce production (via TNF-α-dependent mechanisms) and activation (via secretion of cathepsin L) of heparanase contributed by the colon epithelium. Thus, disruption of the heparanase-driven chronic inflammatory circuit is highly relevant to the design of therapeutic interventions in colitis and the associated cancer.
Figures
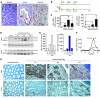
Figure 1. Expression of heparanase and CatL is induced during the course of colitis.
(A) Heparanase expression in acute and chronic phases of UC. Tissue specimens derived from normal colon tissue (left) and UC patients in acute (middle) and chronic (right) phases of the disease were stained with anti-heparanase antibody (red staining). Photographs are representative of control (n = 29) and UC (n = 10) samples (original magnification, ×200). (B) Schematic representation of mouse model of DSS colitis (top) and AOM/DSS-induced colitis–associated carcinoma (bottom), induced as described in Methods. Mouse colonic tissues were harvested at indicated time points and analyzed as described below for C–G. (C) Heparanase (Hpa; left) and CatL (right) mRNA expression during the course of DSS-induced colitis, measured by qRT-PCR and normalized to actin mRNA (n = 5) *P < 0.05, **P < 0.01, ***P < 0.001. (D) Heparanase and CatL protein levels. Lysates of distal colons were harvested at the indicated time points and analyzed for heparanase and CatL protein by immunoblotting. Pro-, latent 65 kDa proenzyme; Active-, enymatically-active 50 kDa heparanase. (E) Densitometric quantification of total (65 kDa and 50 kDa, left) and active (50 kDa, right) heparanase presented in D. (F) Heparanase activity. Punch biopsies were harvested on day 59 from DSS-untreated (Cont) and treated (DSS) mice and cultured for 24 hours. Heparanase activity was determined in the conditioned medium. (G) Immunostaining (brown) of mouse colonic serial tissue sections with anti-heparanase (top) and CatL (bottom) antibodies. Note heparanase staining in the epithelial compartment and CatL staining in the stromal compartment (original magnification, ×200).
Figure 2. Overexpression of heparanase confers increased susceptibility to colitis-associated tumors in AOM/DSS-treated and DSS-treated mice.
(A–F) AOM/DSS-treated mice. (G and H) DSS-treated mice. (A) Gross appearance. Colons (n = 9) were removed on day 110 from AOM/DSS-treated mice, opened longitudinally, washed in PBS, and photographed. (B) Sub-gross examination (original magnification, ×3) and (C) quantification of colonic tumors detected on day 110 (AOM/DSS-treated mice) revealed increased tumor incidence (C, top) and burden (C, bottom) in Hpa Tg colon (gray bars) versus WT colon (black bars) (n = 9). (D) Immunostaining with anti–β-catenin antibody reveals enhanced expression and nuclear localization of β-catenin in Hpa Tg (bottom) versus WT (top) colonic tumors (original magnification, ×400). (E and F) Increased vascular density in Hpa Tg versus WT colonic tumors evaluated by immunostaining with anti-CD31 antibody (original magnification, ×400) (E) and quantified by blind counting of blood vessels per microscopic field (F). (G and H) DSS-induced chronic colitis (without AOM administration) results in colonic tumor formation in Hpa Tg but not in WT mice. (G) Representative microphotographs of histological sections showing the presence of tumors in colons derived from Hpa Tg (bottom) but not WT (top) mice. Magnification, ×100; inset: boxed regions shown at higher magnification (×400). (H) Number of tumors per colon (top) and percentage of tumor-bearing animals (bottom) in Hpa Tg versus WT mice. Error bars represent mean ± SD. *P < 0.05, **P < 0.01.
Figure 3. Heparanase preserves chronic inflammatory conditions in DSS colitis.
Colons were obtained from DSS-treated WT (left) and Hpa Tg (right) mice on day 80 and processed for immunohistochemistry (original magnification, ×200) with anti-heparanase antibody (A); H&E staining (B); immunohistochemistry with antibodies directed against CD31 (C), pIκBα (D), COX-2 (E), and cyclin D1 (G); and immunofluorescence with antibodies directed against pSTAT3 (yellow) (F). Arrows in C indicate CD31-positive microvessels. Cell nuclei were counterstained with DRAQ5 (blue); Scale bars: 25 μm. (A–E and G) Original magnification, ×200. (H) TNF-α secretion by ex vivo–cultured punch biopsies harvested from the colon of untreated and DSS-treated WT (black bars) and Hpa Tg (gray bars) mice on day 80 of the experiment. ND, non-detectable. (I) Increased levels of Tnfa mRNA determined by qRT-PCR in Hpa Tg (gray bars) versus WT (black bars) colon on day 80 of the experiment. Tnfa mRNA levels were normalized to actin mRNA (n = 5). (J) Microvessel counts in colons of Hpa Tg (gray bars) and WT (black bars) mice (n = 6). (K) Number of cyclin D1–positive cells in colons of Hpa Tg (gray bars) and WT (black bars) mice (n = 6). *P < 0.05, ***P < 0.001.
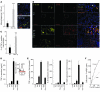
Figure 4. Effect of heparanase on macrophages.
(A) Increased macrophage accumulation during chronic DSS colitis in Hpa Tg colon. Colonic tissue was obtained from Hpa Tg and WT mice on day 80 and stained with anti-F4/80 antibody. F4/80-positive macrophages were quantified per 100-μm2 microscopic field, based on 6 sections from 3 independent mice of each group. Inset: F4/80-positive macrophages (yellow) infiltrating WT (top) and Hpa Tg (bottom) colon. Scale bars: 50 μm. (B) Increased number of TNF-α–expressing macrophages in Hpa Tg colon. Colonic tissue was processed for double immunofluorescent analysis with anti-F4/80 (yellow) and anti–TNF-α (red) antibodies. Cell nuclei were counterstained with DRAQ5 (blue). Scale bars: 50 μm. Insets: Enlarged images of the area delineated by dashed lines. Orange arrows indicate TNF-α–expressing macrophages; white arrowhead indicates TNF-α–negative macrophage. Scale bars: 5 μm. (C) F4/80 and TNF-α double-positive cells (TNF-α–expressing macrophages) were quantified per 100-μm2 microscopic field (6 sections from 3 independent mice), and the percentage of TNF-α–positive macrophages was calculated. (D and E) Heparanase sensitizes macrophages to LPS activation in vitro. Mouse peritoneal macrophages were untreated (Cont) or treated (2 hours, 37°C) with active recombinant heparanase (0.8 mg/ml), LPS (100 ng/ml), heparanase followed by LPS (Hpa+LPS), heat-inactivated heparanase (iHpa) alone, or iHpa followed by LPS (iHpa+LPS). (D) TNF-α secretion was evaluated by ELISA. (E) TNF-α, IL-6, and IL-12p35 expression was assessed by qRT-PCR. (F) Heparanase decreased HS content on macrophage cell surface. RAW264.7 macrophages were either untreated (Cont) or treated (2 hours, 37°C) with active heparanase, and HS content was assessed as described in Methods. ***P < 0.001.
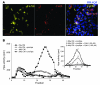
Figure 5. TNF-α induces heparanase expression in Colo205 colonic carcinoma cells and in normal mouse colonic epithelium ex vivo and in vivo.
(A–C) Colo205 cells were incubated (16 hours) in triplicate in the absence or presence of 15 ng/ml TNF-α. Heparanase expression was assessed 16 hours later by (A) immunofluorescent staining (scale bars: 20 μm), (B) qRT-PCR determination of mRNA levels, and (C) enzymatic activity assay. (D and E) Heparanase induction in colonic explants treated with TNF-α ex vivo. Explants harvested from healthy mouse colon as described in Methods were cultured ex vivo for 16 hours in the absence and presence of TNF-α (15 ng/ml). Heparanase expression was assessed 16 hours later by (D) qRT-PCR determination of mRNA levels and (E) immunofluorescent (yellow) staining (scale bars: 50 μm). (F) Induction of heparanase expression in mouse colonic epithelium following TNF-α administration in vivo. Colonic tissue specimens derived from healthy BALB/c mice injected i.p. with PBS or TNF-α (1 μg/mouse, 24 hours) were stained with anti-heparanase antibody (brown staining). Note heparanase staining in colon epithelium derived from TNF-α–treated but not PBS-treated mice (original magnification, ×200).
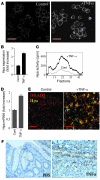
Figure 6. Role of macrophage-derived CatL in proteolytic activation of heparanase.
(A) Colonic tissue was harvested on day 59 and processed for double immunofluorescent analysis applying anti-F4/80 and anti-CatL antibodies. Note that the vast majority of CatL-producing cells are F4/80-positive macrophages. (B) Proteolytic processing and activation of latent heparanase by macrophages. Purified 65-kDa heparanase precursor (proHpa) was incubated with medium conditioned by resting (rMϕ) or LPS-stimulated (sMϕ) mouse peritoneal macrophages. Heparanase enzymatic activity was examined using sulfate-labeled ECM as substrate. Note lack of activity following incubation of the 65-kDa precursor with medium conditioned by rMϕ, as compared with readily detectable activity upon incubation with medium conditioned by sMf. Inset: Proheparanase was incubated with medium conditioned by sMϕ in the absence or presence of increasing concentrations of specific CatL inhibitor I (compound Z-Phe-Phe-CH2F; Calbiochem catalog no. 421419). Heparanase enzymatic activity was examined using sulfate-labeled ECM as substrate.
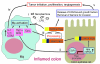
Figure 7. A model of heparanase-driven vicious cycle that powers colitis and the associated tumorigenesis.
(i) Increased levels of TNF-α (secreted, among other cells, by macrophages activated by excessive exposure to the luminal flora, due to epithelial barrier function defects characteristic for UC) induce heparanase expression in colon epithelium via an EGR1-dependent mechanism (ii). (iii) The secreted 65-kDa latent heparanase is processed into its enzymatically active (8 + 50 kDa) form by CatL (which is supplied by the activated macrophages), and in turn sensitizes macrophages to further activation by luminal flora, thus preventing inflammation resolution, switching macrophage responses to the chronic inflammation pattern and creating tumor-inducing inflammatory environment (iv). In addition, heparanase promotes tumor progression via stimulation of angiogenesis, release of ECM-bound growth factors and bioactive HS fragments, and removal of extracellular barriers for invasion (v).
Similar articles
- Heparanase enzyme in chronic inflammatory bowel disease and colon cancer.
Hermano E, Lerner I, Elkin M. Hermano E, et al. Cell Mol Life Sci. 2012 Aug;69(15):2501-13. doi: 10.1007/s00018-012-0930-8. Epub 2012 Feb 14. Cell Mol Life Sci. 2012. PMID: 22331282 Free PMC article. Review. - Macrophage polarization in pancreatic carcinoma: role of heparanase enzyme.
Hermano E, Meirovitz A, Meir K, Nussbaum G, Appelbaum L, Peretz T, Elkin M. Hermano E, et al. J Natl Cancer Inst. 2014 Oct 18;106(12):dju332. doi: 10.1093/jnci/dju332. Print 2014 Dec. J Natl Cancer Inst. 2014. PMID: 25326645 Free PMC article. - Heparanase is preferentially expressed in human psoriatic lesions and induces development of psoriasiform skin inflammation in mice.
Lerner I, Zcharia E, Neuman T, Hermano E, Rubinstein AM, Vlodavsky I, Elkin M. Lerner I, et al. Cell Mol Life Sci. 2014 Jun;71(12):2347-2357. doi: 10.1007/s00018-013-1496-9. Epub 2013 Oct 30. Cell Mol Life Sci. 2014. PMID: 24169805 Free PMC article. - MicroRNA214 Is Associated With Progression of Ulcerative Colitis, and Inhibition Reduces Development of Colitis and Colitis-Associated Cancer in Mice.
Polytarchou C, Hommes DW, Palumbo T, Hatziapostolou M, Koutsioumpa M, Koukos G, van der Meulen-de Jong AE, Oikonomopoulos A, van Deen WK, Vorvis C, Serebrennikova OB, Birli E, Choi J, Chang L, Anton PA, Tsichlis PN, Pothoulakis C, Verspaget HW, Iliopoulos D. Polytarchou C, et al. Gastroenterology. 2015 Oct;149(4):981-92.e11. doi: 10.1053/j.gastro.2015.05.057. Epub 2015 Jun 6. Gastroenterology. 2015. PMID: 26055138 Free PMC article. - Heparanase: From basic research to therapeutic applications in cancer and inflammation.
Vlodavsky I, Singh P, Boyango I, Gutter-Kapon L, Elkin M, Sanderson RD, Ilan N. Vlodavsky I, et al. Drug Resist Updat. 2016 Nov;29:54-75. doi: 10.1016/j.drup.2016.10.001. Epub 2016 Oct 6. Drug Resist Updat. 2016. PMID: 27912844 Free PMC article. Review.
Cited by
- Heparanase in inflammation and inflammation-associated cancer.
Meirovitz A, Goldberg R, Binder A, Rubinstein AM, Hermano E, Elkin M. Meirovitz A, et al. FEBS J. 2013 May;280(10):2307-19. doi: 10.1111/febs.12184. Epub 2013 Mar 4. FEBS J. 2013. PMID: 23398975 Free PMC article. Review. - Versatile role of heparanase in inflammation.
Goldberg R, Meirovitz A, Hirshoren N, Bulvik R, Binder A, Rubinstein AM, Elkin M. Goldberg R, et al. Matrix Biol. 2013 Jun 24;32(5):234-240. doi: 10.1016/j.matbio.2013.02.008. Epub 2013 Mar 13. Matrix Biol. 2013. PMID: 23499528 Free PMC article. Review. - Heparanase is the possible link between monkeypox and Covid-19: robust candidature in the mystic and present perspective.
Al-Kuraishy HM, Al-Gareeb AI, Hetta HF, Alexiou A, Papadakis M, Batiha GE. Al-Kuraishy HM, et al. AMB Express. 2023 Jan 27;13(1):13. doi: 10.1186/s13568-023-01517-y. AMB Express. 2023. PMID: 36705773 Free PMC article. Review. - Targeting Heparanase in Cancer: Inhibition by Synthetic, Chemically Modified, and Natural Compounds.
Mohan CD, Hari S, Preetham HD, Rangappa S, Barash U, Ilan N, Nayak SC, Gupta VK, Basappa, Vlodavsky I, Rangappa KS. Mohan CD, et al. iScience. 2019 May 31;15:360-390. doi: 10.1016/j.isci.2019.04.034. Epub 2019 May 3. iScience. 2019. PMID: 31103854 Free PMC article. Review. - Heparanase in Acute Kidney Injury.
Abassi Z, Goligorsky MS. Abassi Z, et al. Adv Exp Med Biol. 2020;1221:685-702. doi: 10.1007/978-3-030-34521-1_28. Adv Exp Med Biol. 2020. PMID: 32274732 Free PMC article. Review.
References
- Elkin M, et al. Heparanase as mediator of angiogenesis: mode of action. FASEB J. 2001;15(9):1661–1663. - PubMed
- Kato M, et al. Physiological degradation converts the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of FGF-2. Nat Med. 1998;4(6):691–697. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous