Autophagy protects the proximal tubule from degeneration and acute ischemic injury - PubMed (original) (raw)
doi: 10.1681/ASN.2010070705. Epub 2011 Apr 14.
Yoshitsugu Takabatake, Atsushi Takahashi, Jun-ya Kaimori, Isao Matsui, Tomoko Namba, Harumi Kitamura, Fumio Niimura, Taiji Matsusaka, Tomoyoshi Soga, Hiromi Rakugi, Yoshitaka Isaka
Affiliations
- PMID: 21493778
- PMCID: PMC3083312
- DOI: 10.1681/ASN.2010070705
Autophagy protects the proximal tubule from degeneration and acute ischemic injury
Tomonori Kimura et al. J Am Soc Nephrol. 2011 May.
Abstract
Autophagy is a bulk protein degradation system that likely plays an important role in normal proximal tubule function and recovery from acute ischemic kidney injury. Using conditional Atg5 gene deletion to eliminate autophagy in the proximal tubule, we determined whether autophagy prevents accumulation of damaged proteins and organelles with aging and ischemic renal injury. Autophagy-deficient cells accumulated deformed mitochondria and cytoplasmic inclusions, leading to cellular hypertrophy and eventual degeneration not observed in wildtype controls. In autophagy-deficient mice, I/R injury increased proximal tubule cell apoptosis with accumulation of p62 and ubiquitin positive cytoplasmic inclusions. Compared with control animals, autophagy-deficient mice exhibited significantly greater elevations in serum urea nitrogen and creatinine. These data suggest that autophagy maintains proximal tubule cell homeostasis and protects against ischemic injury. Enhancing autophagy may provide a novel therapeutic approach to minimize acute kidney injury and slow CKD progression.
Copyright © 2011 by the American Society of Nephrology
Figures
Figure 1.
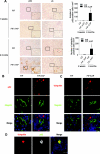
Physiologic changes in proximal tubule-specific autophagy-deficient mice. (A and B) Western blot analysis confirmed Atg5 deficiency, suppression of the conversion of LC3-I to LC3-II, and accumulation of p62 in (A) kidney cortexes of 8-week-old _Atg5_flox/flox; KAP-Cre+ (F/F; KAP) mice (n = 5) and in (B) lysates of isolated proximal tubular cells of 3-week-old _Atg5_flox/flox; KAP-Cre+ mice (n = 3 to 4). A representative immunoblot is shown. The graph shows the ratios of band intensities of Atg5-Atg12, LC3-II, and p62 to those of actin, standardized to the mean of the control. Megalin was blotted as a marker of the proximal tubular cells. (C) Glycosiuria was observed in 6-month-old _Atg5_flox/flox; KAP-Cre+ mice (n = 5 to 6). (D) Mild amino aciduria was observed in 6-month-old _Atg5_flox/flox; KAP-Cre+ mice (n = 5 to 6). Cysteine and serine were not detected. Data are presented as means ± SD. *P < 0.05 _versus Atg5_flox/flox; _KAP-Cre_− mice of same age.
Figure 2.
Histologic changes in proximal tubule-specific autophagy-deficient mice. (A) PAS-stained kidney cortex from 3-week-old, 8-week-old, 6-month-old, and 9-month-old_Atg5_flox/flox; _KAP-Cre_− (left) and _Atg5_flox/flox; KAP-Cre+ (right) mice (n = 4 to 5). _Atg5_flox/flox; KAP-Cre+ mice exhibited slight hypertrophy of the tubular cells at 8 weeks and accumulation of cytosolic amorphous substrates at 6 months. Arrows indicate tubular cells with cytosolic accumulation of amorphous substrates. Bars, 50 and 20 μm (insets). (B) Immunostaining of megalin, a marker of the proximal tubular brush border, showed that the hypertrophied cells (8 weeks) and amorphous substrates-accumulated cells (9 months) are proximal tubular cells in _Atg5_flox/flox; KAP-Cre+ mice (n = 5). Bar, 20 μm. (C–E) Atg5 deficiency causes accumulation of crescent-like structures and deformed mitochondria. Electron micrographs of kidney proximal tubules from _Atg5_flox/flox; _KAP-Cre_− (C and E) and _Atg5_flox/flox; KAP-Cre+ (D, F, and G) mice of 8 weeks (C and D) and 9 months (E through G) of age. The autophagy-deficient proximal tubular cells showed accumulation of crescent-like structures in 8-week-old (D) and 9-month-old (F) mice. (G) The autophagy-deficient proximal tubular cells of 9-month-old mice also contained deformed mitochondria. The figure is a representative of multiple experiments (n = 3). Bars, 2 μm (C–F) and 500 nm (insets of C–F and G). Magnification, ×400 (A), ×1000 (insets of A and B), ×2500 (C through F), and ×10,000 (insets of C–F and G).
Figure 3.
Atg5 deficiency causes accumulation of p62- and ubiquitin-positive inclusions in the kidney cortex tubular cells of 9-month-old mice. (A) Immunohistologic analysis of _Atg5_flox/flox; _KAP-Cre_− and _Atg5_flox/flox; KAP-Cre+ mice kidney cortexes from 8-week-old and 9-month-old mice. Kidney tubular cells of 8-week-old mice showed little staining of p62 (left) or ubiquitin (right), whereas those of 9-month-old mice showed massive accumulation of p62- and ubiquitin -positive inclusions in _Atg5_flox/flox; KAP-Cre+ mice. These inclusions were not obsereved in _Atg5_flox/flox; KAP-Cre_− mice. †_P < 0.05 versus 8-week-old _Atg5_flox/flox; KAP-Cre+ mice; #P < 0.05 versus 9-month-old _Atg5_flox/flox; _KAP-Cre_− mice. Data are presented as means ± SD. (B and C) Immunofluorescence analysis showed that marked accumulation of p62-positive (B) and ubiquitin-positive (C) large dots was observed in autophagy-deficient kidney proximal tubules of 9-month-old mice. Megalin was used as a marker of the proximal tubules. DAPI staining was performed as a counterstaining. (D) p62 and ubiquitin colocalized in the inclusions of autophagy-deficient kidney proximal tubules of 9-month-old mice. The figure is representative of a multiple experiments (n = 5). Bars, 50 (A), 20 (insets of A), 10 (B and C), and 5μm (D). Magnification, ×400 (A), ×1000 (Inlets), ×352 (B and C), and ×1058 (D).
Figure 4.
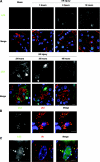
Formation of LC3 dots in kidney proximal tubules of LC3-GFP transgenic mice in response to I/R injury. (A) The presence of LC3-positive dots in the proximal tubules of the kidney of LC3-GFP transgenic mice was examined by immunofluorescence analysis after a sham operation and after 3, 6, 12, 24, 36, and 48 hours after unilateral I/R injury. The number of LC3 dots increased time-dependently after injury for up to 24 hours, after which it decreased. Lotus tetragonolobus lectin (LTA) was used as a marker of proximal tubules, and DAPI staining was performed as a counterstaining. (B and C) LC3 dots colocalized with p62 (B) and ubiquitin dots (C) after I/R injury. Kidney tissues 12 hours after unilateral I/R injury is shown. The figure is representative of a multiple experiments (n = 3 to 5). Bars, 10 (A) and 5 μm (B and C). Magnification, ×352 (A), ×1058 (B), and ×1408 (C).
Figure 5.
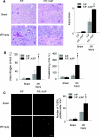
Marked injury in autophagy-deficient kidney cortex after I/R injury. (A) Histologic analysis of _Atg5_flox/flox; _KAP-Cre_− (left) and _Atg5_flox/flox; KAP-Cre+ (right) kidneys 2 days after unilateral I/R injury (n = 5). PAS-stained kidney cortexes showed that the tubules of _Atg5_flox/flox; KAP-Cre+ mice were severely injured compared with I/R-injured _Atg5_flox/flox mice. The tubular injury score of the _Atg5_flox/flox; KAP-Cre+ mice was significantly increased compared with that of controls. (B) Bilateral I/R injury caused significant increase of serum urea nitrogen and creatinine in _Atg5_flox/flox; KAP-Cre+ mice compared with I/R-injured control littermates (n = 4 to 6). (C) Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling staining 2 days after I/R injury showed increased number of apoptotic tubular cells in the cortex of Atg5_flox/flox; KAP-Cre+ mice (n = 5). ‡_P < 0.05 versus sham-operated Atg5_flox/flox; KAP-Cre+ mice; §_P < 0.05 versus I/R injury-operated _Atg5_flox/flox; _KAP-Cre_− mice. Data are presented as means ± SD. Bars: 100 (A) and 10 μm (C). The images are representatives of multiple experiments. Magnification, ×200 (A) and ×352 (C).
Figure 6.
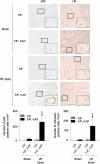
Accumulation of p62- and ubiquitin-positive inclusions in the autophagy-deficient kidney cortex after I/R injury. Immunohistologic analysis of _Atg5_flox/flox; _KAP-Cre_− and _Atg5_flox/flox; KAP-Cre+ kidneys 2 days after I/R injury. p62 (left) or ubiquitin (right) staining of kidney cortexes showed massive accumulation of p62- and ubiquitin-positive inclusions in Atg5_flox/flox; KAP-Cre+ kidneys after I/R injury. ‡_P < 0.05 versus sham-operated Atg5_flox/flox; KAP-Cre+ mice; §_P < 0.05 versus I/R injury-operated _Atg5_flox/flox; _KAP-Cre_− mice. Data are presented as means ± SD. The figure is a representative of multiple experiments (n = 5). Bars, 50 and 20 μm (insets). Magnification, ×400 and ×1000 (insets).
Figure 7.
Formation of p62- and ubiquitin-positive inclusions in autophagy-deficient kidney proximal tubules in response to I/R injury. (A and B) Immunofluorescence analysis showed that marked accumulation of p62-positive large dots (A) and ubiquitin-positive dots (B) was observed in autophagy-deficient kidney proximal tubules 2 days after I/R injury. (C) p62 and ubiquitin colocalized in the inclusions of autophagy-deficient kidney proximal tubules 2 days after I/R injury. Megalin was stained as a marker of proximal tubules in A and B. The figure is a representative of multiple experiments (n = 5). Bars, 10 (A and B) and 5 μm (C). Magnification, ×352 (A and B) and ×1058 (C).
Similar articles
- Autophagy in proximal tubules protects against acute kidney injury.
Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Jiang M, et al. Kidney Int. 2012 Dec;82(12):1271-83. doi: 10.1038/ki.2012.261. Epub 2012 Aug 1. Kidney Int. 2012. PMID: 22854643 Free PMC article. - Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys.
Livingston MJ, Wang J, Zhou J, Wu G, Ganley IG, Hill JA, Yin XM, Dong Z. Livingston MJ, et al. Autophagy. 2019 Dec;15(12):2142-2162. doi: 10.1080/15548627.2019.1615822. Epub 2019 May 22. Autophagy. 2019. PMID: 31066324 Free PMC article. - The protective role of autophagy against aging and acute ischemic injury in kidney proximal tubular cells.
Isaka Y, Kimura T, Takabatake Y. Isaka Y, et al. Autophagy. 2011 Sep;7(9):1085-7. doi: 10.4161/auto.7.9.16465. Epub 2011 Sep 1. Autophagy. 2011. PMID: 21606682 - Maladaptive proximal tubule repair: cell cycle arrest.
Bonventre JV. Bonventre JV. Nephron Clin Pract. 2014;127(1-4):61-4. doi: 10.1159/000363673. Epub 2014 Sep 24. Nephron Clin Pract. 2014. PMID: 25343823 Review. - Autophagy in acute kidney injury.
Livingston MJ, Dong Z. Livingston MJ, et al. Semin Nephrol. 2014 Jan;34(1):17-26. doi: 10.1016/j.semnephrol.2013.11.004. Epub 2013 Nov 21. Semin Nephrol. 2014. PMID: 24485026 Free PMC article. Review.
Cited by
- mTORC1 and mTORC2 Co-Protect against Cadmium-Induced Renal Tubular Epithelial Cell Apoptosis and Acute Kidney Injury by Regulating Protein Kinase B.
Zhu J, Gong Z, Wang X, Zhang K, Ma Y, Zou H, Song R, Zhao H, Liu Z, Dong W. Zhu J, et al. J Agric Food Chem. 2024 Sep 11;72(36):19667-19679. doi: 10.1021/acs.jafc.4c05702. Epub 2024 Sep 1. J Agric Food Chem. 2024. PMID: 39219293 - The connection between autophagy and ferroptosis in AKI: recent advances regarding selective autophagy.
Pan C, Zhao H, Cai X, Wu M, Qin B, Li J. Pan C, et al. Ren Fail. 2024 Dec;46(2):2379601. doi: 10.1080/0886022X.2024.2379601. Epub 2024 Aug 4. Ren Fail. 2024. PMID: 39099238 Free PMC article. Review. - Deciphering the neuroprotective mechanisms of RACK1 in cerebral ischemia-reperfusion injury: Pioneering insights into mitochondrial autophagy and the PINK1/Parkin axis.
Zhao L, Chen Y, Li H, Ding X, Li J. Zhao L, et al. CNS Neurosci Ther. 2024 Aug;30(8):e14836. doi: 10.1111/cns.14836. CNS Neurosci Ther. 2024. PMID: 39097918 Free PMC article. - Kidney Injury in a Murine Hemorrhagic Shock/Resuscitation Model Is Alleviated by sulforaphane's Anti-Inflammatory and Antioxidant Action.
Li Y, Qin K, Liang W, Yan W, Fragoulis A, Pufe T, Buhl EM, Zhao Q, Greven J. Li Y, et al. Inflammation. 2024 Jul 18. doi: 10.1007/s10753-024-02106-2. Online ahead of print. Inflammation. 2024. PMID: 39023831 - Clinical characteristics and treatment compounds of obesity-related kidney injury.
Mao TH, Huang HQ, Zhang CH. Mao TH, et al. World J Diabetes. 2024 Jun 15;15(6):1091-1110. doi: 10.4239/wjd.v15.i6.1091. World J Diabetes. 2024. PMID: 38983811 Free PMC article. Review.
References
- Mizushima N, Ohsumi Y, Yoshimori T: Autophagosome formation in mammalian cells. Cell Struct Funct 27: 421–429, 2002 - PubMed
- Levine B, Klionsky DJ: Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6: 463–477, 2004 - PubMed
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N: Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441: 885–889, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases