Endothelial progenitor dysfunction in the pathogenesis of diabetic retinopathy: treatment concept to correct diabetes-associated deficits - PubMed (original) (raw)
Endothelial progenitor dysfunction in the pathogenesis of diabetic retinopathy: treatment concept to correct diabetes-associated deficits
Sergio Li Calzi et al. EPMA J. 2010.
Abstract
Progressive obliteration of the retinal microvessels is a characteristic of diabetic retinopathy and the resultant retinal ischemia can lead to sight-threatening macular edema, macular ischemia and ultimately preretinal neovascularization. Bone marrow derived endothelial progenitor cells (EPCs) play a critical role in vascular maintenance and repair. There is still great debate about the most appropriate markers that define an EPC. EPCs can be isolated using cell sorting by surface phenotype selection or in vitro cell culture. For freshly isolated cells, EPC cell sorting is heavily dependent on the surface markers used; EPCs can also be isolated by in vitro propagation of heterogeneous mixtures of cells in culture using adhesion to specific substrates and cell growth characteristics. in vitro isolation enables consistent reproducibility and using this approach at least two distinct types of EPCs with different angiogenic properties have been identified from adult peripheral and umbilical cord blood; early EPCs (eEPCs) and late outgrowth endothelial progenitor cells (OECs). Emerging studies demonstrate the potential of these cells in revascularization of ischemic/injured retina in animal models of retinal disease. Since ischemic retinopathies are leading causes of blindness, they are a potential disease target for EPC-based therapy. In this chapter, we summarize the current knowledge about EPCs and discuss the possibility of cellular therapy for treatment of diabetic macular ischemia and the vasodegenerative phase of diabetic retinopathy. We also report current pharmacological options that can be utilized to correct diabetes associated defects in EPCs so as to enhance the therapeutic utility of these cells.
Figures
Fig. 1
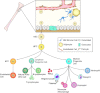
Adult stem cells of the bone marrow. The bone marrow hosts at least two known types of adult stem cells, the mesenchymal stem cells (MSC) and the hematopoietic stem cells (HSC); the most prominent adult stem cell in the bone marrow. The HSC can give rise to the hematopoietic progenitor cells (HPC) which in turn give rise to the lymphoid progenitor cell, the myeloid progenitor cells, and likely the EPC. The precise origin of the EPC is under debate as this cell may directly arise from the HSC or from the HPC. The bone marrow microenvironment is composed of bone marrow stromal cells (which are the source of SDF-1), adipocytes, and cells of the bone matrix, osteoblasts and osteoclasts. The vessels within the bone marrow, composed of pericytes and endothelium, function to provide a barrier between the hematopoietic compartment and the circulatory system. Figure adapted from Domen, et al. [5]
Fig. 2
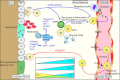
Mobilization and homing are two closely related processes. Mobilization involves the exodus of HSC/HPC from the bone marrow into the circulation while homing is the “opposite” of this event. HSC mobilize from the endosteal niche, move to the vascular niche, and ultimately into the circulation. This normally occurs when stress induces changes of SDF-1 levels in the bone marrow. The mechanism of stress-induced mobilization as occurs following irradiation or G-CSF-induced mobilization is not fully known, but is, in part, accomplished by the upregulation of proteases such as MMP-2, MMP-9, cathepsin-G and elastase. These proteases cleave niche retention signals like membrane-bound stem cell factor (mSCF), SDF-1, VCAM-1 and osteopontin (Opn). Gradients of fibroblast growth factor 4 (FGF-4) also regulate mobilization. For homing events, key steps are needed. Upon reaching the bone marrow vasculature, SDF-1-stimulated circulating HSC/HPC express integrins such as very late antigen 4 (VLA-4) and hyaluronan binding–cellular adhesion molecule (CD44). These integrins, in turn, interact with vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), E-and P-selectins expressed on bone marrow endothelial cells which slows down the circulating HSC/HSP in a process known as “rolling.” Following rolling, firm adhesion and subsequent endothelia transmigration into the hematopoietic compartment is mainly accomplished by VLA-4 interactions. Once extravasated the cells, the cells migrate along extravascular hematopoietic cords toward specific niches through as SDF-1 gradient or receding oxygen gradient originating from the supporting osteoblastic or endothelia niches. BMEC: Bone marrow microvascular endothelial cell. Figure adapted from Wilson and Trumpp [10]
Fig. 3
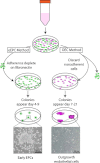
Common methods of precursor isolation. Culture of eEPCs includes a 5-day process wherein non-adherent MNCs give rise to the EPC colony. OECs are derived from adherent MNCs cultured on collagen for 21 days in endothelial growth conditions and demonstrate typical cobblestone morphology
Fig. 4
Morphology of Early and Late Outgrowth EPCs. (A) Typical colony of early EPCs which develop into spindle shaped cells by day 7 (B). Early EPCs do not proliferate readily, which leads to the typical sub-confluent appearance. In contrast, late outgrowth ECs (C) show rapid growth as indicated by the dividing cell (black arrow) and display the typical endothelial cobblestone morphology and proliferate abundantly (D)
Fig. 5
Schematic of the hypothetic diabetic retinopathy progress. In physiological conditions, CD34+ EPCs contribute to routine blood vessel maintenance through eNOS activation and NO-mediated stimulation of CD14+ EPCs. In diabetes, initially, cytokines like stem cell factor (SCF), monocyte chemoattractant protein-1 (MCP-1), interleukin-8 (IL-8), and tumor necrosis factor alpha (TNF-α) released by dysfunctional CD34+ EPCs initiate CD14+ EPC-mediated aberrant vascular repair resulting in retinal ischemia. This phase is referred to as non proliferative diabetic retinopathy (NPDR). The vasodegenerative phase of diabetic retinopathy associated with reduced reparative function of EPCs evolves in the proliferative diabetic retinopathy (PDR). This phase is characterized by pathological neovascularization seen in the diabetic retina
Fig. 6
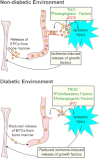
Release of EPCs is reduced in a diabetic environment. In a non-diabetic environment ischemic injury results in the release of growth factors at the site of injury which stimulate the release of EPCs from bone marrow. EPCs then migrate to the site of injury and initiate blood vessel repair (angiogenesis). In a diabetic environment ROS, proinflamatory and antiangiogenic factors are increased above non-diabetic levels while NO is reduced. This results in a blunted response to ischemic injury and marginal repair at the site of injury
Similar articles
- Promise of endothelial progenitor cell for treatment of diabetic retinopathy.
Bhatwadekar AD, Shaw LC, Grant MB. Bhatwadekar AD, et al. Expert Rev Endocrinol Metab. 2010 Jan;5(1):29-37. doi: 10.1586/eem.09.75. Expert Rev Endocrinol Metab. 2010. PMID: 23678364 Free PMC article. - Outgrowth endothelial cells: characterization and their potential for reversing ischemic retinopathy.
Medina RJ, O'Neill CL, Humphreys MW, Gardiner TA, Stitt AW. Medina RJ, et al. Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5906-13. doi: 10.1167/iovs.09-4951. Epub 2010 Jun 16. Invest Ophthalmol Vis Sci. 2010. PMID: 20554606 - Endothelial progenitor cells (EPCs) mobilized and activated by neurotrophic factors may contribute to pathologic neovascularization in diabetic retinopathy.
Liu X, Li Y, Liu Y, Luo Y, Wang D, Annex BH, Goldschmidt-Clermont PJ. Liu X, et al. Am J Pathol. 2010 Jan;176(1):504-15. doi: 10.2353/ajpath.2010.081152. Epub 2009 Nov 30. Am J Pathol. 2010. PMID: 19948824 Free PMC article. - Dysfunction and Therapeutic Potential of Endothelial Progenitor Cells in Diabetes Mellitus.
Hu L, Dai SC, Luan X, Chen J, Cannavicci A. Hu L, et al. J Clin Med Res. 2018 Oct;10(10):752-757. doi: 10.14740/jocmr3581w. Epub 2018 Sep 10. J Clin Med Res. 2018. PMID: 30214646 Free PMC article. Review. - Advances in cell therapies using stem cells/progenitors as a novel approach for neurovascular repair of the diabetic retina.
Lechner J, Medina RJ, Lois N, Stitt AW. Lechner J, et al. Stem Cell Res Ther. 2022 Jul 30;13(1):388. doi: 10.1186/s13287-022-03073-x. Stem Cell Res Ther. 2022. PMID: 35907890 Free PMC article. Review.
Cited by
- Endothelial Progenitor Cells and Cardiovascular Correlates.
Mudyanadzo TA. Mudyanadzo TA. Cureus. 2018 Sep 21;10(9):e3342. doi: 10.7759/cureus.3342. Cureus. 2018. PMID: 30473975 Free PMC article. Review. - Birth asphyxia as the major complication in newborns: moving towards improved individual outcomes by prediction, targeted prevention and tailored medical care.
Golubnitschaja O, Yeghiazaryan K, Cebioglu M, Morelli M, Herrera-Marschitz M. Golubnitschaja O, et al. EPMA J. 2011 Jun;2(2):197-210. doi: 10.1007/s13167-011-0087-9. Epub 2011 Jun 9. EPMA J. 2011. PMID: 23199149 Free PMC article. - Molecular imaging of retinal disease.
Capozzi ME, Gordon AY, Penn JS, Jayagopal A. Capozzi ME, et al. J Ocul Pharmacol Ther. 2013 Mar;29(2):275-86. doi: 10.1089/jop.2012.0279. Epub 2013 Feb 19. J Ocul Pharmacol Ther. 2013. PMID: 23421501 Free PMC article. Review. - Photobiomodulation Mitigates Diabetes-Induced Retinopathy by Direct and Indirect Mechanisms: Evidence from Intervention Studies in Pigmented Mice.
Saliba A, Du Y, Liu H, Patel S, Roberts R, Berkowitz BA, Kern TS. Saliba A, et al. PLoS One. 2015 Oct 1;10(10):e0139003. doi: 10.1371/journal.pone.0139003. eCollection 2015. PLoS One. 2015. PMID: 26426815 Free PMC article. - Mesenchymal stem cells-microvesicle-miR-451a ameliorate early diabetic kidney injury by negative regulation of P15 and P19.
Zhong L, Liao G, Wang X, Li L, Zhang J, Chen Y, Liu J, Liu S, Wei L, Zhang W, Lu Y. Zhong L, et al. Exp Biol Med (Maywood). 2018 Nov;243(15-16):1233-1242. doi: 10.1177/1535370218819726. Epub 2019 Jan 6. Exp Biol Med (Maywood). 2018. PMID: 30614256 Free PMC article.
References
- ETDRS Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:766–85. - PubMed
- ETDRS Focal photocoagulation treatment of diabetic macular edema. ETDRS Report Number 19. Arch Ophthalmol. 1995;113:1144–55. - PubMed
- Domen J, Wagers A, Weissman IL. Bone Marrow (hematiopoietic) Stem Cells [Online]. 2006.
LinkOut - more resources
Full Text Sources