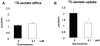Acute regulation of cardiac metabolism by the hexosamine biosynthesis pathway and protein O-GlcNAcylation - PubMed (original) (raw)
Acute regulation of cardiac metabolism by the hexosamine biosynthesis pathway and protein O-GlcNAcylation
Boglárka Laczy et al. PLoS One. 2011.
Abstract
Objective: The hexosamine biosynthesis pathway (HBP) flux and protein O-linked N-acetyl-glucosamine (O-GlcNAc) levels have been implicated in mediating the adverse effects of diabetes in the cardiovascular system. Activation of these pathways with glucosamine has been shown to mimic some of the diabetes-induced functional and structural changes in the heart; however, the effect on cardiac metabolism is not known. Therefore, the primary goal of this study was to determine the effects of glucosamine on cardiac substrate utilization.
Methods: Isolated rat hearts were perfused with glucosamine (0-10 mM) to increase HBP flux under normoxic conditions. Metabolic fluxes were determined by (13)C-NMR isotopomer analysis; UDP-GlcNAc a precursor of O-GlcNAc synthesis was assessed by HPLC and immunoblot analysis was used to determine O-GlcNAc levels, phospho- and total levels of AMPK and ACC, and membrane levels of FAT/CD36.
Results: Glucosamine caused a dose dependent increase in both UDP-GlcNAc and O-GlcNAc levels, which was associated with a significant increase in palmitate oxidation with a concomitant decrease in lactate and pyruvate oxidation. There was no effect of glucosamine on AMPK or ACC phosphorylation; however, membrane levels of the fatty acid transport protein FAT/CD36 were increased and preliminary studies suggest that FAT/CD36 is a potential target for O-GlcNAcylation.
Conclusion/interpretation: These data demonstrate that acute modulation of HBP and protein O-GlcNAcylation in the heart stimulates fatty acid oxidation, possibly by increasing plasma membrane levels of FAT/CD36, raising the intriguing possibility that the HBP and O-GlcNAc turnover represent a novel, glucose dependent mechanism for regulating cardiac metabolism.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
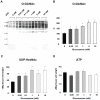
Figure 1. Effect of glucosamine on A, B) Overall cardiac O-GlcNAc levels; C) UDP-HexNAc concentrations and D) ATP concentrations.
* P<0.05 vs. 0 mM, one-way ANOVA with Dunnett's posthoc test. Western blots: 0 mM (n = 8), 0.05 mM (n = 5), 0.1 mM (n = 9), 1 mM (n = 4), 5 mM (n = 8), 10 mM (n = 7). HPLC: 0 mM (n = 4), 0.05 mM (n = 5), 0.1 mM (n = 5), 1 mM (n = 4), 5 mM (n = 3), 10 mM (n = 3). Note that equal protein loading for the O-GlcNAc immunoblots was assessed by Sypro staining and overall O-GlcNAc levels were normalized to untreated control group.
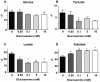
Figure 2. Effect of glucosamine on A) glucose; B) pyruvate; C) lactate and D) palmitate oxidation.
* P<0.05 vs. 0 mM glucosamine, one-way ANOVA with Dunnett's posthoc test. 0 mM (n = 6), 0.05 mM (n = 4), 0.1 mM (n = 5), 5 mM (n = 5), 10 mM (n = 4).
Figure 3. Effect of 0.1 mM glucosamine on A) unlabeled glycolytic lactate efflux and B) exogenous [3-13C]lactate uptake * P<0.05 vs. 0 mM, Student's t-test.
0 mM (n = 4), 0.1 mM (n = 5).
Figure 4. A) AMPK and B) ACC phosphorylation in the heart after 60 min perfusion with 0.1 mM glucosamine.
0 mM (n = 4), 0.1 mM (n = 5).
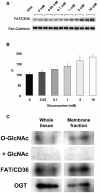
Figure 5. A) Immunoblots of Plasma membrane fraction for FAT/CD36 following 60 min perfusion with 0, 0.05, 0.1, 1, 5 and 10 mM; pan-cadherin included as a plasma membrane marker and protein loading control; B) Densitometric analysis of FAT/CD36 immunoblots normalized to 0 mM glucosamine; P<0.05 vs. 0 mM, one-way ANOVA with Dunnett's posthoc test; n = 2 in each group; C) Immunoprecipitation of FAT/CD36 from whole tissue and plasma membrane lysates, followed by O-GlcNAc and OGT immunoblots.
Specificity of O-GlcNAc antibody was confirmed by co-incubation with 10 mM N-acetylglucosamine (GlcNAc).
Similar articles
- First characterization of glucose flux through the hexosamine biosynthesis pathway (HBP) in ex vivo mouse heart.
Olson AK, Bouchard B, Zhu WZ, Chatham JC, Des Rosiers C. Olson AK, et al. J Biol Chem. 2020 Feb 14;295(7):2018-2033. doi: 10.1074/jbc.RA119.010565. Epub 2020 Jan 8. J Biol Chem. 2020. PMID: 31915250 Free PMC article. - Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia.
Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Liu J, et al. J Mol Cell Cardiol. 2006 Feb;40(2):303-12. doi: 10.1016/j.yjmcc.2005.11.003. Epub 2005 Dec 9. J Mol Cell Cardiol. 2006. PMID: 16337959 - Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels.
Liu J, Marchase RB, Chatham JC. Liu J, et al. J Mol Cell Cardiol. 2007 Jan;42(1):177-85. doi: 10.1016/j.yjmcc.2006.09.015. Epub 2006 Oct 27. J Mol Cell Cardiol. 2007. PMID: 17069847 Free PMC article. - The Hexosamine Biosynthesis Pathway: Regulation and Function.
Paneque A, Fortus H, Zheng J, Werlen G, Jacinto E. Paneque A, et al. Genes (Basel). 2023 Apr 18;14(4):933. doi: 10.3390/genes14040933. Genes (Basel). 2023. PMID: 37107691 Free PMC article. Review. - Hexosamine biosynthesis and protein O-glycosylation: the first line of defense against stress, ischemia, and trauma.
Chatham JC, Nöt LG, Fülöp N, Marchase RB. Chatham JC, et al. Shock. 2008 Apr;29(4):431-40. doi: 10.1097/shk.0b013e3181598bad. Shock. 2008. PMID: 17909453 Review.
Cited by
- Nonsyndromic X-linked intellectual deficiency in three brothers with a novel MED12 missense mutation [c.5922G>T (p.Glu1974His)].
Bouazzi H, Lesca G, Trujillo C, Alwasiyah MK, Munnich A. Bouazzi H, et al. Clin Case Rep. 2015 Jul;3(7):604-9. doi: 10.1002/ccr3.301. Epub 2015 May 26. Clin Case Rep. 2015. PMID: 26273451 Free PMC article. - O-GlcNAcylation: cellular physiology and therapeutic target for human diseases.
Ye L, Ding W, Xiao D, Jia Y, Zhao Z, Ao X, Wang J. Ye L, et al. MedComm (2020). 2023 Dec 19;4(6):e456. doi: 10.1002/mco2.456. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38116061 Free PMC article. Review. - O-GlcNAcylation of AMPA receptor GluA2 is associated with a novel form of long-term depression at hippocampal synapses.
Taylor EW, Wang K, Nelson AR, Bredemann TM, Fraser KB, Clinton SM, Puckett R, Marchase RB, Chatham JC, McMahon LL. Taylor EW, et al. J Neurosci. 2014 Jan 1;34(1):10-21. doi: 10.1523/JNEUROSCI.4761-12.2014. J Neurosci. 2014. PMID: 24381264 Free PMC article. - Metabolic effects of glutamine on the heart: anaplerosis versus the hexosamine biosynthetic pathway.
Lauzier B, Vaillant F, Merlen C, Gélinas R, Bouchard B, Rivard ME, Labarthe F, Dolinsky VW, Dyck JR, Allen BG, Chatham JC, Des Rosiers C. Lauzier B, et al. J Mol Cell Cardiol. 2013 Feb;55:92-100. doi: 10.1016/j.yjmcc.2012.11.008. Epub 2012 Nov 28. J Mol Cell Cardiol. 2013. PMID: 23201305 Free PMC article. - Cardiac Molecular Analysis Reveals Aging-Associated Metabolic Alterations Promoting Glycosaminoglycans Accumulation via Hexosamine Biosynthetic Pathway.
Grilo LF, Zimmerman KD, Puppala S, Chan J, Huber HF, Li G, Jadhav AYL, Wang B, Li C, Clarke GD, Register TC, Oliveira PJ, Nathanielsz PW, Olivier M, Pereira SP, Cox LA. Grilo LF, et al. Adv Sci (Weinh). 2024 Oct;11(38):e2309211. doi: 10.1002/advs.202309211. Epub 2024 Aug 9. Adv Sci (Weinh). 2024. PMID: 39119859 Free PMC article.
References
- An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2006;291:H1489–1506. - PubMed
- Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. - PubMed
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle: Its role in insulin sensitivity and metabolic disturbances of diabetes mellitus. The Lancet. 1963;1:785–789. - PubMed
- Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. - PubMed
- Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–3317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL067464/HL/NHLBI NIH HHS/United States
- R01 HL079364/HL/NHLBI NIH HHS/United States
- R01 HL101192/HL/NHLBI NIH HHS/United States
- HL067464/HL/NHLBI NIH HHS/United States
- HL101192/HL/NHLBI NIH HHS/United States
- 9575/CAPMC/ CIHR/Canada
- HL079364/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous