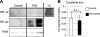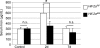Intestinal hypoxia-inducible factor-2alpha (HIF-2alpha) is critical for efficient erythropoiesis - PubMed (original) (raw)
Intestinal hypoxia-inducible factor-2alpha (HIF-2alpha) is critical for efficient erythropoiesis
Erik R Anderson et al. J Biol Chem. 2011.
Abstract
Erythropoiesis is a coordinated process by which RBCs are produced. Erythropoietin, a kidney-derived hormone, and iron are critical for the production of oxygen-carrying mature RBCs. To meet the high demands of iron during erythropoiesis, small intestinal iron absorption is increased through an undefined mechanism. In this study, erythropoietic induction of iron absorption was further investigated. Hypoxia-inducible factor-2α (HIF-2α) signaling was activated in the small intestine during erythropoiesis. Genetic disruption of HIF-2α in the intestine abolished the increase in iron absorption genes as assessed by quantitative real-time reverse transcription-PCR and Western blot analyses. Moreover, the increase in serum iron following induction of erythropoiesis was entirely dependent on intestinal HIF-2α expression. Complete blood count analysis demonstrated that disruption of intestinal HIF-2α inhibited efficient erythropoiesis; mice disrupted for HIF-2α demonstrated lower hematocrit, RBCs, and Hb compared with wild-type mice. These data further cement the essential role of HIF-2α in regulating iron absorption and also demonstrate that hypoxia sensing in the intestine, as well as in the kidney, is essential for regulation of erythropoiesis by HIF-2α.
Figures
FIGURE 1.
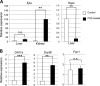
PhZ-induced hemolysis activates expression of EPO in the kidney and iron absorption genes in the small intestine. Wild-type mice were injected twice 24 h apart with 60 mg of PhZ/kg of body weight or with normal saline (Control) and killed 2 days later. qPCR was performed in the kidney or liver for Epo and hepcidin (Hepc) (A) or in the duodenum for Dmt1, Dcytb, and Fpn1 (B). Expression was normalized to β-actin. Values are expressed as -fold change compared with untreated controls. Four to five animals for each treatment group were assessed. Statistical analyses were performed using Student's t test. Each bar represents the mean ± S.D. **, p < 0.01; ***, p < 0.005; n.s., not significant.
FIGURE 2.
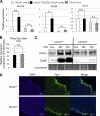
Erythropoietic induction of iron absorption genes in the small intestine is HIF-2α-dependent. Wild-type (_Hif-2_αF/F) and intestinal HIF-2 knock-out (_Hif-2_αΔIE) mice were treated with PhZ or saline (control) and killed 48 h post-treatment. qPCR was performed in the duodenum for Dmt1, Dcytb, and Fpn1 (A) or in the kidney for Epo (B). Expression was normalized to β-actin. Values are expressed as -fold change compared with untreated controls. C, _Hif-2_αF/F and _Hif-2_αΔIE mice were treated with PhZ or saline (control (Con)) and killed 48 and 72 h post-treatment. Western blot analysis measuring Dmt1a and DcytB expression in membrane extracts and Coomassie Blue staining of total membrane proteins were assessed for loading controls. D, immunofluorescence staining of Fpn1 in the duodenums of _Hif-2_αF/F and _Hif-2_αΔIE mice following PhZ treatment. Four to five animals for each treatment group were assessed. Statistical analyses were performed using Student's t test. Each bar represents the mean ± S.D. *, p < 0.05; **, p < 0.01; n.s., not significant. For Western blot analysis and immunofluorescence, a representative image from an individual mouse for each treatment group or time point is shown.
FIGURE 3.
PhZ treatment induces HIF-2α (but not HIF-1α) expression in the small intestine. A, Western blot analysis of duodenal nuclear extracts for HIF-1α and HIF-2α following PhZ or saline (Control) treatment. Hypoxia-treated Caco-2 cells were used as a positive control (+C) for HIF-1α. Expression was normalized to RAN protein expression. B, tissue non-heme iron quantification of the duodenums of PhZ- and saline-treated mice. Four to five animals for each treatment group were assessed. For Western blot analysis, representative images from two individual mice for each treatment group are shown. n.s., not significant.
FIGURE 4.
HIF-2α is stabilized by hypoxia in the small intestine following PhZ treatment. A, qPCR analysis of duodenal Hif-2α mRNA expression following PhZ or saline (Control) treatment in wild-type mice. B, Western blot analysis of Caco-2 cells treated with recombinant human EPO under normoxia and hypoxia for 24 h. Expression was normalized to GAPDH protein expression. C, Western blot analysis of nitroimidazole-protein adducts in extracts from the duodenums and lungs of PhZ-treated and control (Con) wild-type mice. Expression was normalized to GAPDH. Four to five animals for each treatment group were assessed. For Western blot analysis, a representative image from an individual mouse for each treatment group is shown. n.s., not significant.
FIGURE 5.
Intestinal HIF-2 expression is critical for the erythropoietic increase in serum iron. Wild-type (_Hif-2_αF/F) and intestinal HIF-2 knock-out (_Hif-2_αΔIE) mice were treated with PhZ or saline (Control), and sera were collected 2 (2d) and 7 (7d) days after the last injection. Serum was assayed for iron content. Four to five animals for each treatment group were assessed. Statistical analyses were performed using Student's t test. Error bars indicate S.D. *, p < 0.05; n.s., not significant.
FIGURE 6.
Intestinal HIF-2 expression is essential for erythropoiesis. Wild-type (_Hif-2_αF/F) and intestinal HIF-2 knock-out (_Hif-2_αΔIE) mice were treated with PhZ or saline (Control), and blood was collected 2 (2d) and 7 (7d) days after the last injection. Complete blood count analysis was performed measuring RBCs (A), HCT (B), and Hb (C). Four to five animals for each treatment group were assessed. Statistical analyses were performed using Student's t test. Error bars indicate S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.005; n.s., not significant.
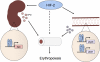
FIGURE 7.
HIF-2 is a central regulator of erythropoiesis. Following hemolysis, the kidney and intestine experience hypoxia due to poor oxygen delivery. In the kidney, HIF-2α is stabilized, allowing it to bind to and activate the Epo promoter. In the small intestine, HIF-2α activates the Dcytb and Dmt1 genes, increasing iron absorption. Increased levels of circulating EPO and iron stimulate erythropoiesis and supply new erythrocytes with iron for hemoglobin production. ARNT, aryl hydrocarbon receptor nuclear translocator.
Similar articles
- Intestine-specific Disruption of Hypoxia-inducible Factor (HIF)-2α Improves Anemia in Sickle Cell Disease.
Das N, Xie L, Ramakrishnan SK, Campbell A, Rivella S, Shah YM. Das N, et al. J Biol Chem. 2015 Sep 25;290(39):23523-7. doi: 10.1074/jbc.C115.681643. Epub 2015 Aug 19. J Biol Chem. 2015. PMID: 26296885 Free PMC article. - The IRP1-HIF-2α axis coordinates iron and oxygen sensing with erythropoiesis and iron absorption.
Anderson SA, Nizzi CP, Chang YI, Deck KM, Schmidt PJ, Galy B, Damnernsawad A, Broman AT, Kendziorski C, Hentze MW, Fleming MD, Zhang J, Eisenstein RS. Anderson SA, et al. Cell Metab. 2013 Feb 5;17(2):282-90. doi: 10.1016/j.cmet.2013.01.007. Cell Metab. 2013. PMID: 23395174 Free PMC article. - HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice.
Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. Mastrogiannaki M, et al. J Clin Invest. 2009 May;119(5):1159-66. doi: 10.1172/JCI38499. Epub 2009 Apr 6. J Clin Invest. 2009. PMID: 19352007 Free PMC article. - Regulation of erythropoiesis by hypoxia-inducible factors.
Haase VH. Haase VH. Blood Rev. 2013 Jan;27(1):41-53. doi: 10.1016/j.blre.2012.12.003. Epub 2013 Jan 3. Blood Rev. 2013. PMID: 23291219 Free PMC article. Review. - Hypoxia-inducible factors link iron homeostasis and erythropoiesis.
Shah YM, Xie L. Shah YM, et al. Gastroenterology. 2014 Mar;146(3):630-42. doi: 10.1053/j.gastro.2013.12.031. Epub 2013 Dec 31. Gastroenterology. 2014. PMID: 24389303 Free PMC article. Review.
Cited by
- Activation of Intestinal HIF2α Ameliorates Iron-Refractory Anemia.
Yu Y, Su Y, Yang S, Liu Y, Lin Z, Das NK, Wu Q, Zhou J, Sun S, Li X, Yue W, Shah YM, Min J, Wang F. Yu Y, et al. Adv Sci (Weinh). 2024 Mar;11(12):e2307022. doi: 10.1002/advs.202307022. Epub 2024 Jan 20. Adv Sci (Weinh). 2024. PMID: 38243847 Free PMC article. - Editorial: New insights into dyserythropoiesis: from pathophysiology, molecular mechanisms to treatments for erythroid disorders.
Zhang S, Mei Y, Zhao B. Zhang S, et al. Front Cell Dev Biol. 2023 Nov 2;11:1321170. doi: 10.3389/fcell.2023.1321170. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38020925 Free PMC article. No abstract available. - High-Altitude Hypoxia Induces Excessive Erythrocytosis in Mice via Upregulation of the Intestinal HIF2a/Iron-Metabolism Pathway.
Zhou S, Yan J, Song K, Ge RL. Zhou S, et al. Biomedicines. 2023 Nov 7;11(11):2992. doi: 10.3390/biomedicines11112992. Biomedicines. 2023. PMID: 38001992 Free PMC article. - Myeloid Hif2α is not essential to maintain systemic iron homeostasis.
Jain C, Parimi S, Huang W, Hannifin S, Singhal R, Das NK, Lee KE, Shah YM. Jain C, et al. Exp Hematol. 2023 Sep-Oct;125-126:25-36.e1. doi: 10.1016/j.exphem.2023.08.001. Epub 2023 Aug 8. Exp Hematol. 2023. PMID: 37562670 Free PMC article. - Updated perspective of EPAS1 and the role in pulmonary hypertension.
Wang N, Hua J, Fu Y, An J, Chen X, Wang C, Zheng Y, Wang F, Ji Y, Li Q. Wang N, et al. Front Cell Dev Biol. 2023 Feb 27;11:1125723. doi: 10.3389/fcell.2023.1125723. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36923253 Free PMC article. Review.
References
- Corwin H. L. (2006) Transfus. Med. Rev. 20, 27–33 - PubMed
- Anderson G. J., Powell L. W., Halliday J. W. (1990) Gastroenterology 98, 576–585 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical