Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis - PubMed (original) (raw)
. 2011 Apr 15;25(8):831-44.
doi: 10.1101/gad.615311.
Romain H Fontaine, Jihane Soueid, Tuomas Tammela, Taija Makinen, Clara Alfaro-Cervello, Fabien Bonnaud, Andres Miguez, Lucile Benhaim, Yunling Xu, Maria-José Barallobre, Imane Moutkine, Johannes Lyytikkä, Turgut Tatlisumak, Bronislaw Pytowski, Bernard Zalc, William Richardson, Nicoletta Kessaris, Jose Manuel Garcia-Verdugo, Kari Alitalo, Anne Eichmann, Jean-Léon Thomas
Affiliations
- PMID: 21498572
- PMCID: PMC3078708
- DOI: 10.1101/gad.615311
Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis
Charles-Félix Calvo et al. Genes Dev. 2011.
Abstract
Neural stem cells (NSCs) are slowly dividing astrocytes that are intimately associated with capillary endothelial cells in the subventricular zone (SVZ) of the brain. Functionally, members of the vascular endothelial growth factor (VEGF) family can stimulate neurogenesis as well as angiogenesis, but it has been unclear whether they act directly via VEGF receptors (VEGFRs) expressed by neural cells, or indirectly via the release of growth factors from angiogenic capillaries. Here, we show that VEGFR-3, a receptor required for lymphangiogenesis, is expressed by NSCs and is directly required for neurogenesis. Vegfr3:YFP reporter mice show VEGFR-3 expression in multipotent NSCs, which are capable of self-renewal and are activated by the VEGFR-3 ligand VEGF-C in vitro. Overexpression of VEGF-C stimulates VEGFR-3-expressing NSCs and neurogenesis in the SVZ without affecting angiogenesis. Conversely, conditional deletion of Vegfr3 in neural cells, inducible deletion in subventricular astrocytes, and blocking of VEGFR-3 signaling with antibodies reduce SVZ neurogenesis. Therefore, VEGF-C/VEGFR-3 signaling acts directly on NSCs and regulates adult neurogenesis, opening potential approaches for treatment of neurodegenerative diseases.
Figures
Figure 1.
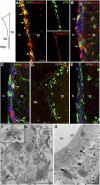
Vegfr3:YFP expression in the adult SVZ. (Top left panel) The localization of Vegfr3:YFP cells (green) around the lv is schematically represented. All images are taken in the lateral ventricular wall facing the striatum (St). Confocal (A–E) and electron microscopy (F,G) images from Vegfr3:YFP mouse brain vibratome sections are stained with Abs against cell type-specific markers as indicated. (A) Vegfr3:YFP cells are immunolabeled by anti-VEGFR-3 Ab (yellow arrows), indicating that YFP faithfully reports VEGFR-3 expression in SVZ cells. (B) Vegfr3:YFP cells are a subpopulation of GFAP+ cells (yellow arrows), extending long cytoplasmic processes into the SVZ. (C) Few Vegfr3:YFP cells are labeled by anti-EGFR Ab (yellow arrow), but most of the EGFR+ cells are YFP-negative. YFP is expressed by few vessels in the striatum outside the SVZ (white arrow). (D) The majority of BrdU LRCs along the striatal SVZ are Vegfr3:YFP cells (yellow arrows). (E) Vegfr3:YFP does not label DCX+ neuroblasts (red). (White arrow) Blood vessel. (F,G) Immunogold labeling of Vegfr3:YFP cells in the ventricular zone–SVZ. (F) A labeled type B1 NSC extending a primary cilium (magnification in inset). (G) A blood vessel (bv, YFP-negative) is enwrapped by the cytoplasmic process of a Vegfr3:YFP astrocyte (asterisk). (Arrows) Basal laminae. Bars: A–E, 10 μm; F, 2 μm; G, 500 nm.
Figure 2.
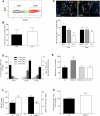
Isolation of NSCs from adult Vegfr3:YFP mice. (A) YFP-positive and YFP-negative cells from the periventricular zone of Vegfr3:YFP mice were FACS-sorted. (B) The cells were tested for their ability to generate primary neurospheres in the presence of bFGF/EGF (2 × 104 cells per well in 24-well plates). Histograms show that YFP-positive and YFP-negative cells form primary neurospheres and include a subset of 0.5% of cell-forming spheres (n = 6). (C) YFP-positive and YFP-negative cells from primary neurospheres are multipotent, differentiating into TuJ1+ neurons, GFAP+ astrocytes, and O4+ oligodendrocytes after growth factor removal. Quantification is shown in the histogram (n = 3). (D) YFP+/EGFR+, YFP+/EGFR−, YFP−/EGFR+, and YFP−/EGFR− subpopulations isolated after labeling with EGF-647 are tested for their capacity to self-renew and generate secondary (2ary NS) and tertiary (3ary NS) neurospheres. Histogram shows the increase in the number of neurosphere cells (NS-cells) relative to the number of plated cells. Only EGFR+/VEGFR-3+ cells generate numerous secondary neurospheres (2ary NS) and tertiary neurospheres (3ary NS) (n = 4), even forming neurospheres after six passages (6ary NS) (data not shown), and thus correspond to the majority of NSCs. (E) Primary neurospheres cultured in the presence of VEGF-C (50 ng/mL) and 31-C1 (a mouse VEGFR-3 function-blocking Ab, 5 μg/mL). The number of neurosphere formed (NS formed) is expressed as a percentage of the control. The 31-C1 Ab blocks the VEGF-C-induced amplification of neurospheres (n = 3). (F) Dissociated neurosphere cells are counted and TUNEL-labeled in control or VEGF-C-containing (50 ng/mL) medium (n = 3). (G) Increased proliferation of YFP+/EGFR+ NSCs following VEGF-C treatment (50 ng/mL). Cells were pulsed with BrdU (10 μM) for 24 h and fixed after 2 d in vitro (n = 3). Bar: C, 20 μm. Error bars indicate SEM. (*) P < 0.05; (***) P < 0.001, Student's _t_-test.
Figure 3.
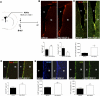
In vivo VEGF-C overexpression. (A) Adult mice are infected with either AAV-VEGF-C or control AAVs (AAV-ctl: AAV-EGFP and AAV-HSA) in the vicinity of the SVZ (coordinates from the bregma: anterior, 0.5; lateral, 1.0; depth, 2.1; n = 7 animals per group) and injected intraperitoneally with BrdU the same day. (B) Coronal brain cryosections from animals sacrificed at day 2 post-infection are analyzed for the number of BrdU cells (red) in the SVZ. Both the dorsolateral and ventral regions along the striatal wall show more BrdU+ cells in AAV-VEGF-C-treated animals as compared with AAV-ctl. Histograms indicate the number of newborn BrdU+ cells (bottom left) and activated Caspase-3+ cells (bottom right) in the SVZ. AAV-VEGF-C promotes the production of newborn cells and inhibits PCD in SVZ cells. (C) Coronal cryosections of the periventricular zone immunolabeled with anti-DCX Ab (green). In the striatal SVZ, DCX labeling reflects neuroblast production. AAV-VEGF-C induces a significant expansion of DCX expression in the SVZ compared with controls. (D,E) Coronal brain vibratome sections from Vegfr3:YFP adult mice infected with either AAV-VEGF-C or AAV-ctl and sacrificed at day 2. Dividing VEGFR-3-expressing cells (BrdU+YFP+) are increased in AAV-VEGF-C-treated animals (D), while the subventricular expressions of GFAP and YFP are also enhanced (E), as compared with controls.(St) Striatum. Bars: B,C, 100 μm; D,E, 20 μm. Error bars indicate SEM. (***) P < 0.001, Student's _t_-test.
Figure 4.
Effects of AAV-VEGF-A and AAV-VEGF-C overexpression on the adult SVZ. Blood vessel pattern in the striatal wall, as shown by labeling of endothelial cells (CD31, green) and pericytes (PDGFR-β, red), while astrocytes are stained with anti-GFAP Ab (blue). AAV-VEGF-C-treated and AAV-VEGF-C156-treated (a VEGF-C variant that cannot bind to VEGFR-2) animals display a vascular network similar to AAV-ctl. In contrast, AAV-VEGF-A induced robust angiogenesis, which is attested to by a dense network of CD31+ endothelial cells and PDGFRβ+ pericytes at the site of injection. Note that the number of astroglial cells increased in AAV-VEGF-C- and AAV-VEGF-C156-treated animals compared with controls. Bar, 20 μm.
Figure 5.
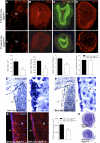
Genetic and pharmacological loss of VEGFR-3 function in vivo. (A–D) Neonatal neurogenesis in P15 Brn4:Cre, Vegfr3lox/lox mice. Brn4:Cre, Vegfr3lox/lox mice show a decreased number of BrdU+ cells in the lv (A) and OB (B), as compared with Brn4:Cre, Vegfr3lox/+ controls. (C) The OB is reduced in size and is disorganized, with a reduction of NeuN+ cells. (D) No decrease of the density of CD31 labeling was observed. (E–G) SVZ of adult mice with a conditional deletion of Vegfr3 in neural cells (Brn4:Cre, Vegfr3lox/lox homozygotes; control Brn4:Cre, Vegfr3lox/+ heterozygotes). (E,F) Cresyl-violet staining of brain coronal paraffin sections from control (E) and _Vegfr3_-deficient (F) mice. The SVZ cell density along the striatal wall and in the lateral horn is reduced in Vegfr3lox/lox homozygotes compared with controls. Insets show magnifications of boxed areas in E and F. (G) Anti-GFAP staining indicates that the SVZ astroglial network is thinner and disorganized in Vegfr3lox/lox homozygotes compared with controls. (H) Cresyl-violet staining of OB coronal paraffin sections shows the reduction in OB size of _Vegfr3_-deficient mice compared with heterozygote control. Bars: A, 50 μm; B–F,H, 100 μm; G, 20 μm; insets in E,F, 5 μm. Error bars indicate SEM. (*) P < 0.05, Student's _t_-test.
Figure 6.
(A) Function-blocking Abs against VEGFR-3 (31-C1: anti-VEGFR-3) or rat IgG2a (Ig-ctl, control) were delivered above the lv of adult Vegfr3:YFP mice with an Alzet miniosmotic pump for 6 d (coordinates from the bregma: anterior, 0.5; lateral, 1.0; depth, 0). Coronal brain vibratome sections are stained with specific markers as indicated (left panels), and histograms show the corresponding cell quantifications (right panels). Anti-VEGFR-3-treated mice display few BrdU+ cells and reduced expression patterns for GFAP and YFP in the SVZ, as compared with control animals. (B) Tx-induced deletion of Vegfr3 in subventricular astroglial cells in GlastCreERT2:Cre, Vegfr3lox/lox mice. Coronal cryosection of the adult lv walls showing reduction in the expression of GFAP and DCX, and in the number of dividing KI67+ cells. No changes were observed in the pattern of CD31+ endothelial cells. Quantifications confirm a phenotype similar to the one observed in Brn4:Cre, Vegfr3lox/lox mice. Bars: A, 20 μm; B, 50 μm. n = 3–4 animals per group. Error bars indicate SEM. (*) P < 0.05; (**) P ≤ 0.005, Student's _t_-test.
Figure 7.
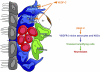
Proposed model for VEGF-C and VEGFR-3 function in the adult SVZ. VEGFR-3 is expressed by a subpopulation of astrocytes (B) and almost all NSCs (B1), but not by the majority of TAPs (C), neuroblasts (A), and endothelial cells (bv). EGFR is expressed by the subpopulation of VEGFR-3 NSCs. VEGF-C produced by SVZ astrocytes and also other cell types promotes activation of VEGFR-3-expressing cells (i.e., niche astrocytes and NSCs), which increase in number following overexpression of VEGF-C. This enhances the number of TAPs and neuroblasts.
Similar articles
- Vascular endothelial growth factor C stimulates progression of human gastric cancer via both autocrine and paracrine mechanisms.
Kodama M, Kitadai Y, Tanaka M, Kuwai T, Tanaka S, Oue N, Yasui W, Chayama K. Kodama M, et al. Clin Cancer Res. 2008 Nov 15;14(22):7205-14. doi: 10.1158/1078-0432.CCR-08-0818. Clin Cancer Res. 2008. PMID: 19010837 - Vascular endothelial growth factor receptor 3 controls neural stem cell activation in mice and humans.
Han J, Calvo CF, Kang TH, Baker KL, Park JH, Parras C, Levittas M, Birba U, Pibouin-Fragner L, Fragner P, Bilguvar K, Duman RS, Nurmi H, Alitalo K, Eichmann AC, Thomas JL. Han J, et al. Cell Rep. 2015 Feb 24;10(7):1158-72. doi: 10.1016/j.celrep.2015.01.049. Epub 2015 Feb 19. Cell Rep. 2015. PMID: 25704818 Free PMC article. - The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis.
Heckman CA, Holopainen T, Wirzenius M, Keskitalo S, Jeltsch M, Ylä-Herttuala S, Wedge SR, Jürgensmeier JM, Alitalo K. Heckman CA, et al. Cancer Res. 2008 Jun 15;68(12):4754-62. doi: 10.1158/0008-5472.CAN-07-5809. Cancer Res. 2008. PMID: 18559522 - AIP1 mediates vascular endothelial cell growth factor receptor-3-dependent angiogenic and lymphangiogenic responses.
Zhou HJ, Chen X, Huang Q, Liu R, Zhang H, Wang Y, Jin Y, Liang X, Lu L, Xu Z, Min W. Zhou HJ, et al. Arterioscler Thromb Vasc Biol. 2014 Mar;34(3):603-15. doi: 10.1161/ATVBAHA.113.303053. Epub 2014 Jan 9. Arterioscler Thromb Vasc Biol. 2014. PMID: 24407031 Free PMC article. - VEGFs and receptors involved in angiogenesis versus lymphangiogenesis.
Lohela M, Bry M, Tammela T, Alitalo K. Lohela M, et al. Curr Opin Cell Biol. 2009 Apr;21(2):154-65. doi: 10.1016/j.ceb.2008.12.012. Epub 2009 Feb 21. Curr Opin Cell Biol. 2009. PMID: 19230644 Review.
Cited by
- Semaphorin3A, Neuropilin-1, and PlexinA1 are required for lymphatic valve formation.
Bouvrée K, Brunet I, Del Toro R, Gordon E, Prahst C, Cristofaro B, Mathivet T, Xu Y, Soueid J, Fortuna V, Miura N, Aigrot MS, Maden CH, Ruhrberg C, Thomas JL, Eichmann A. Bouvrée K, et al. Circ Res. 2012 Aug 3;111(4):437-45. doi: 10.1161/CIRCRESAHA.112.269316. Epub 2012 Jun 21. Circ Res. 2012. PMID: 22723296 Free PMC article. - Interactions between VEGFR and Notch signaling pathways in endothelial and neural cells.
Thomas JL, Baker K, Han J, Calvo C, Nurmi H, Eichmann AC, Alitalo K. Thomas JL, et al. Cell Mol Life Sci. 2013 May;70(10):1779-92. doi: 10.1007/s00018-013-1312-6. Epub 2013 Mar 12. Cell Mol Life Sci. 2013. PMID: 23479133 Free PMC article. Review. - Engineered 3D vascular and neuronal networks in a microfluidic platform.
Osaki T, Sivathanu V, Kamm RD. Osaki T, et al. Sci Rep. 2018 Mar 26;8(1):5168. doi: 10.1038/s41598-018-23512-1. Sci Rep. 2018. PMID: 29581463 Free PMC article. - High gene expression levels of VEGFA and CXCL8 in the peritumoral brain zone are associated with the recurrence of glioblastoma: A bioinformatics analysis.
Luo X, Xu S, Zhong Y, Tu T, Xu Y, Li X, Wang B, Yang F. Luo X, et al. Oncol Lett. 2019 Dec;18(6):6171-6179. doi: 10.3892/ol.2019.10988. Epub 2019 Oct 14. Oncol Lett. 2019. PMID: 31788092 Free PMC article. - Vascular Platform to Define Hematopoietic Stem Cell Factors and Enhance Regenerative Hematopoiesis.
Poulos MG, Crowley MJP, Gutkin MC, Ramalingam P, Schachterle W, Thomas JL, Elemento O, Butler JM. Poulos MG, et al. Stem Cell Reports. 2015 Nov 10;5(5):881-894. doi: 10.1016/j.stemcr.2015.08.018. Epub 2015 Oct 1. Stem Cell Reports. 2015. PMID: 26441307 Free PMC article.
References
- Acker T, Beck H, Plate KH 2001. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascularization. Mech Dev 108: 45–57 - PubMed
- Bock F, Onderka J, Dietrich T, Bachmann B, Pytowski B, Cursiefen C 2008. Blockade of VEGFR3-signalling specifically inhibits lymphangiogenesis in inflammatory corneal neovascularisation. Graefes Arch Clin Exp Ophthalmol 246: 115–119 - PubMed
- Carmeliet P 2005. Angiogenesis in life, disease and medicine. Nature 438: 932–936 - PubMed
- Cesetti T, Obernier K, Bengtson CP, Fila T, Mandl C, Holzl-Wenig G, Worner K, Eckstein V, Ciccolini F 2009. Analysis of stem cell lineage progression in the neonatal subventricular zone identifies EGFR+/NG2− cells as transit-amplifying precursors. Stem Cells 27: 1443–1454 - PubMed
- Chojnacki AK, Mak GK, Weiss S 2009. Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both? Nat Rev Neurosci 10: 153–163 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous