Munc13 mediates the transition from the closed syntaxin-Munc18 complex to the SNARE complex - PubMed (original) (raw)
Munc13 mediates the transition from the closed syntaxin-Munc18 complex to the SNARE complex
Cong Ma et al. Nat Struct Mol Biol. 2011 May.
Abstract
During the priming step that leaves synaptic vesicles ready for neurotransmitter release, the SNARE syntaxin-1 transitions from a closed conformation that binds Munc18-1 tightly to an open conformation within the highly stable SNARE complex. Control of this conformational transition is important for brain function, but the underlying mechanism is unknown. NMR and fluorescence experiments now show that the Munc13-1 MUN domain, which plays a central role in vesicle priming, markedly accelerates the transition from the syntaxin-1-Munc18-1 complex to the SNARE complex. This activity depends on weak interactions of the MUN domain with the syntaxin-1 SNARE motif, and probably with Munc18-1. Together with available physiological data, these results provide a defined molecular basis for synaptic vesicle priming, and they illustrate how weak protein-protein interactions can play crucial biological roles by promoting transitions between high-affinity macromolecular assemblies.
Figures
Figure 1
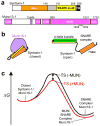
Weak interactions can dramatically accelerate transitions between tight complexes. (a) Domain diagrams of rat syntaxin-1A and Munc13-1. Residue numbers indicate selected domain boundaries. The position of the loop sequence removed in MUN* (residues 1408–1452) is also indicated. (b) Diagrams of the closed syntaxin-1–Munc18-1 complex [Munc18-1 in purple; syntaxin-1 regions colored as in panel a] and the SNARE complex [only the SNARE motifs are shown for synaptobrevin (red) and SNAP-25 (green)]. (c) Free energy diagram illustrating how the energy barrier for the transition from the closed syntaxin-1–Munc18-1 complex to the SNARE complex (with Munc18-1 bound to it) could be decreased through weak interactions with the MUN domain, which might lead to the formation of MUN–SNARE complex–Munc18-1 assemblies.
Figure 2
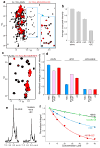
Formation of MUN*-SNARE complex-Munc18-1 macromolecular assemblies. (a) Superposition of expansions of 1H-13C HMQC spectra of 12 μM 2H-13CH3-MUN* before and after addition of 20 μM Munc18-1 (M18) and 20 μM SNARE complex (SC). The inset was plotted at lower contour levels to show that binding did not induce large cross-peak shifts. (b) Average decrease in the intensities of well-resolved cross-peaks from 1H-13C HMQC spectra of 2H-13CH3-MUN* upon addition of 20 μM Munc18-1, 20 μM SNARE complex or both. (c) Superposition of expansions of 1H-13C HMQC spectra of 15 μM 2H-13CH3-SNARE complex before or after addition of 20 μM Munc18-1 and 30 μM MUN*. The 2H-13CH3-SNARE complex was formed with 2H-Ile-13CH3-syntaxin-1(2–253), 2H-Val,Leu-13CH3-synaptobrevin(29–93), 2H- SNAP-25(11–82) and 2H-SNAP-25(141–203). (d) Average decrease in the intensities of well- resolved cross-peaks from 1H-13C HMQC spectra of 15 μM 2H-13CH3-SNARE complex upon addition of 20 μM Munc18-1, 30 μM MUN*, or both. Color-coded bars represent data obtained for cross-peaks from the syntaxin-1 Habc domain (Habc), from the syntaxin-1 and synaptobrevin SNARE motifs (SNARE motif), or both (all). In (b, d) the error bars denote the standard error calculated from consecutive spectra acquired on the same samples. (e) 1D 1H-15N HSQC spectra of 20 μM 15N-MUN* before and after addition of 40 μM Munc18-1 and SNARE complex. (f) Plots of normalized integrals of the amide region of 1D 1H-15N HSQC spectra of 20 μM 15N-labeled MUN* upon addition of different concentrations of Munc18-1, syntaxin-1(2–253)–Munc18-1 complex (Syx/M18)), SNARE complex and Munc18-1 plus SNARE complex. Curves show the fits obtained with a standard one-to-one protein-ligand binding model.
Figure 3
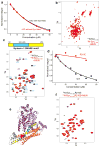
MUN* binds to the syntaxin-1 SNARE motif. (a) Plots of normalized integrals of the amide region of 1D 1H-15N HSQC spectra of 20 μM 15N-SNARE complex containing syntaxin-1(2–253) (SC with Syx Habc) or syntaxin-1(191–253) (SC without Syx Habc) upon addition of different concentrations of MUN*. (b) 2D 1H-15N HSQC spectra of 20 μM 15N-syntaxin-1 N-terminal region (residues 2-180) before or after addition of 30 μM MUN*. (c) 2D 1H-15N HSQC spectra of 20 μM 15N-syntaxin-1 SNARE motif (residues 191–253) before or after addition of 30μM MUN*. Cross-peaks that are strongly broadened by MUN* binding are labeled with their corresponding residue number. The diagram above shows in blue the location of the MUN*-binding region (exhibiting strong broadening) within the syntaxin-1–SNARE motif, including selected residue numbers above. (d) Plots of normalized integrals of the amide region of 1D 1H-15N HSQC spectra of 10 μM wild type (WT) or R210E mutant (R210E) 15N-syntaxin-1 SNARE motif upon addition of different concentrations of MUN*. In (a,d), curves show the fits obtained with a standard one-to-one protein-ligand binding model. (e) Ribbon diagram of the structure of the syntaxin-1 Munc18-1 complex. Munc18-1 is in purple and syntaxin-1 in orange (Habc domain), gray (linker) and yellow (SNARE motif), except that residues that exhibit strong broadening upon binding of MUN* to the isolated syntaxin-1 SNARE motif (see panel c) are in blue. Arg210 is represented by pink hard spheres. The ribbon diagram was generated with Pymol (DeLano Scientific). (f) 2D 1H-15N HSQC spectra of 20 μM 15N-syntaxin-1-R210E SNARE motif before or after addition of 30 μM MUN*.
Figure 4
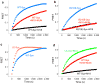
The MUN domain accelerates the transition from the syntaxin-1–Munc18-1 complex to the SNARE complex. (a) Proposed model whereby the MUN domain (pink) promotes the transition from the syntaxin-1–Munc18-1 complex to the SNARE complex through its weak interactions with the syntaxin-1 SNARE motif (color-coding as in Fig. 1b). (b) Superposition of expansions of 1H-13C HMQC spectra of 15 μM 2H-Ile-13CH3-syntaxin-1(2–253) bound to Munc18-1 or incorporated into SNARE complex formed with 2H-Val,Leu-13CH3-synaptobrevin(29–93), 2H-SNAP-25(11–82) and 2H-SNAP-25(141–203). The cross-peak assignments that are available are indicated with the residue number; H3 identifies cross-peaks that are known to belong to the SNARE motif but do not have residue-specific assignment; * indicates tentative assignments (Supplementary Methods). (c-d) 1H-13C HMQC spectra of samples of 15 μM 2H-Ile-13CH3-syntaxin-1(2–253) bound to Munc18-1 acquired immediately after addition of 15 μM 2H-Val,Leu-13CH3-synaptobrevin(29–93), 25 μM 2H-SNAP-25(11–82) and 25 μM 2H-SNAP-25(141–203), in the absence (c) or presence of 30 μM MUN* (d). (e-f) The progress of the transition from the syntaxin-1–Munc18-1 complex to the SNARE complex as a function of time was monitored through the intensity of the disappearing Ile203 cross-peak of the syntaxin-1–Munc18-1 complex and the intensity of the appearing Ile203 cross-peak of the SNARE complex in consecutive 1H-13C HMQC spectra (2.3 hr each) obtained after mixing samples as in (c-d), in the absence (e) or presence (f) of 30 μM MUN*. The vertical axis represents the fraction of reaction completed based on normalized cross-peak intensities (see Supplementary Methods). (g) Progress of the transition from the syntaxin-1–Munc18-1 complex to the SNARE complex in the presence of 30 μM MUN* monitored as in panel (f) but acquiring 10 min 1H-13C HMQC spectra. In (e-g), the curves show the fits of the data to an exponential rise to a maximum.
Figure 5
MUN* activity depends on interactions with the syntaxin-1 SNARE motif and is bypassed by the syntaxin-1 LE mutation. (a-d) SNARE complex assembly reactions monitored by the time-dependent development of FRET between a BODIPY fluorescence donor on residue 61 of synaptobrevin(29–93) and a Rhodamine fluorescence acceptor on residue 249 of syntaxin-1(2–253). Reactions were started by adding 2 μM labeled synaptobrevin, and 10 μM SNAP-25(11–82) and SNAP-25(141–203) to 10 μM WT syntaxin-1(2–253) or the R210E or LE syntaxin-1(2–253) mutants alone or bound to Munc18-1 (+M18) in the absence or in the presence of 30 μM MUN* (+MUN), as indicated.
Similar articles
- Munc13-1 MUN domain and Munc18-1 cooperatively chaperone SNARE assembly through a tetrameric complex.
Shu T, Jin H, Rothman JE, Zhang Y. Shu T, et al. Proc Natl Acad Sci U S A. 2020 Jan 14;117(2):1036-1041. doi: 10.1073/pnas.1914361117. Epub 2019 Dec 30. Proc Natl Acad Sci U S A. 2020. PMID: 31888993 Free PMC article. - Munc13 activates the Munc18-1/syntaxin-1 complex and enables Munc18-1 to prime SNARE assembly.
Wang X, Gong J, Zhu L, Wang S, Yang X, Xu Y, Yang X, Ma C. Wang X, et al. EMBO J. 2020 Aug 17;39(16):e103631. doi: 10.15252/embj.2019103631. Epub 2020 Jul 9. EMBO J. 2020. PMID: 32643828 Free PMC article. - Tomosyn guides SNARE complex formation in coordination with Munc18 and Munc13.
Li Y, Wang S, Li T, Zhu L, Ma C. Li Y, et al. FEBS Lett. 2018 Apr;592(7):1161-1172. doi: 10.1002/1873-3468.13018. Epub 2018 Mar 13. FEBS Lett. 2018. PMID: 29485200 - Molecular Mechanisms Underlying Neurotransmitter Release.
Rizo J. Rizo J. Annu Rev Biophys. 2022 May 9;51:377-408. doi: 10.1146/annurev-biophys-111821-104732. Epub 2022 Feb 15. Annu Rev Biophys. 2022. PMID: 35167762 Free PMC article. Review. - Neuronal SNARE complex assembly guided by Munc18-1 and Munc13-1.
Wang S, Ma C. Wang S, et al. FEBS Open Bio. 2022 Nov;12(11):1939-1957. doi: 10.1002/2211-5463.13394. Epub 2022 Mar 22. FEBS Open Bio. 2022. PMID: 35278279 Free PMC article. Review.
Cited by
- Syntaxin-1 N-peptide and Habc-domain perform distinct essential functions in synaptic vesicle fusion.
Zhou P, Pang ZP, Yang X, Zhang Y, Rosenmund C, Bacaj T, Südhof TC. Zhou P, et al. EMBO J. 2013 Jan 9;32(1):159-71. doi: 10.1038/emboj.2012.307. Epub 2012 Nov 27. EMBO J. 2013. PMID: 23188083 Free PMC article. - A lever hypothesis for Synaptotagmin-1 action in neurotransmitter release.
Jaczynska K, Esser V, Xu J, Sari L, Lin MM, Rosenmund C, Rizo J. Jaczynska K, et al. bioRxiv [Preprint]. 2024 Jun 18:2024.06.17.599417. doi: 10.1101/2024.06.17.599417. bioRxiv. 2024. PMID: 38948826 Free PMC article. Updated. Preprint. - The Sec1/Munc18 protein Vps45 holds the Qa-SNARE Tlg2 in an open conformation.
Eisemann TJ, Allen F, Lau K, Shimamura GR, Jeffrey PD, Hughson FM. Eisemann TJ, et al. Elife. 2020 Aug 17;9:e60724. doi: 10.7554/eLife.60724. Elife. 2020. PMID: 32804076 Free PMC article. - Syntaxin 12 and COMMD3 are new factors that function with VPS33B in the biogenesis of platelet α-granules.
Ambrosio AL, Febvre HP, Di Pietro SM. Ambrosio AL, et al. Blood. 2022 Feb 10;139(6):922-935. doi: 10.1182/blood.2021012056. Blood. 2022. PMID: 34905616 Free PMC article. - Molecular encoding and synaptic decoding of context during salt chemotaxis in C. elegans.
Hiroki S, Yoshitane H, Mitsui H, Sato H, Umatani C, Kanda S, Fukada Y, Iino Y. Hiroki S, et al. Nat Commun. 2022 May 27;13(1):2928. doi: 10.1038/s41467-022-30279-7. Nat Commun. 2022. PMID: 35624091 Free PMC article.
References
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. - PubMed
- Brunger AT. Structure and function of SNARE and SNARE-interacting proteins. Q Rev Biophys. 2005:1–47. - PubMed
- Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases