ARFGAP1 promotes AP-2-dependent endocytosis - PubMed (original) (raw)
doi: 10.1038/ncb2221. Epub 2011 Apr 17.
Helge Gad, Gabriele Turacchio, Emanuele Cocucci, Jia-Shu Yang, Jian Li, Galina V Beznoussenko, Zhongzhen Nie, Ruibai Luo, Lianwu Fu, James F Collawn, Tomas Kirchhausen, Alberto Luini, Victor W Hsu
Affiliations
- PMID: 21499258
- PMCID: PMC3087831
- DOI: 10.1038/ncb2221
ARFGAP1 promotes AP-2-dependent endocytosis
Ming Bai et al. Nat Cell Biol. 2011 May.
Abstract
COPI (coat protein I) and the clathrin-AP-2 (adaptor protein 2) complex are well-characterized coat proteins, but a component that is common to these two coats has not been identified. The GTPase-activating protein (GAP) for ADP-ribosylation factor 1 (ARF1), ARFGAP1, is a known component of the COPI complex. Here, we show that distinct regions of ARFGAP1 interact with AP-2 and coatomer (components of the COPI complex). Selectively disrupting the interaction of ARFGAP1 with either of these two coat proteins leads to selective inhibition in the corresponding transport pathway. The role of ARFGAP1 in AP-2-regulated endocytosis has mechanistic parallels with its roles in COPI transport, as both its GAP activity and coat function contribute to promoting AP-2 transport.
Figures
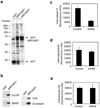
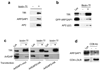
Figure 1. Interactions with ARFGAP1 and effects of its knockdown
a. Pull-down assay detects proteins interacting with ARFGAP1. ARFGAP1 as a GST fusion protein was bound to glutathione beads, incubated with cytosol, and then analyzed for associated proteins by Coomassie staining. b. Pull-down assay detects ARFGAP1 interacting with coat components. ARFGAP1 as a GST fusion protein was bound to glutathione beads, incubated with cytosol, and then immunoblotted for proteins as indicated. ARFGAP1 interacts with components of AP-2, and also with previously known interacting proteins that are components of the COPI complex. c. Tf uptake is reduced by siRNA against ARFGAP1. BSC-1 cells were bound with fluorescence-conjugated Tf, and then assessed for the level of internalized Tf at 10 minutes. The mean from three experiments with standard error is shown. Difference between the two conditions is significant (p<0.05). d. EGF uptake is not markedly affected by siRNA against ARFGAP1. BSC-1 cells were with fluorescence-conjugated EGF, and then assessed for the level of internalized EGF at 10 minutes. The mean from three experiments with standard error is shown. Difference between the two conditions is insignificant (p>0.05). e. LDL uptake is not markedly affected by siRNA against ARFGAP1. BSC-1 cells were bound with fluorescence-conjugated LDL, and then assessed for the level of internalized LDL at 10 minutes. The mean from three experiments with standard error is shown. Difference between the two conditions is insignificant (p>0.05).
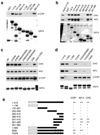
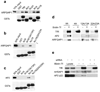
Figure 2. Distinct requirements for ARFGAP1 binding to coatomer versus AP-2 and clathrin
a. Interaction of different truncation mutants of ARFGAP1 with coatomer. The different forms of ARFGAP1 as GST fusion proteins were bound to beads, incubated with cytosol, and then immunoblotted for proteins as indicated. GST fusion proteins were detected by Coomassie staining. b. Interaction of different truncation mutants of ARFGAP1 with AP-2 or clathrin. Pulldown experiments were performed as described above. c. Interaction of different point mutants of ARFGAP1 with AP-2, clathrin, or coatomer. Pulldown experiments were performed as described above. d. Interaction of additional mutants of ARFGAP1 with AP-2, clathrin, or coatomer. Pulldown experiments were performed as described above. e. Interactions of ARFGAP1 with AP-2, clathrin and coatomer are summarized. The catalytic domain of ARFGAP1 is shown in white. Asterisks indicate W-box motifs.
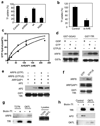
Figure 3. Disrupting interaction with either coatomer or AP-2 leads to selective disruption in transport pathways
a. Pulldown assays to assess direct binding of ARFGAP1 to AP-2. The different forms of ARFGAP1 as GST fusion proteins were bound to beads, incubated with purified AP-2 adaptors, and then immunoblotted for proteins as indicated. GST fusion proteins were detected by Coomassie staining. b. Pulldown assays to assess direct binding of ARFGAP1 to clathrin. The different forms of ARFGAP1 as GST fusion proteins were bound to beads, incubated with purified clathrin triskelia, and then immunoblotted for proteins as indicated. GST fusion proteins were detected by Coomassie staining. c. Sequential incubations reveal that the AP-2 adaptor is needed to link ARFGAP1 to the clathrin triskelion. The different forms of ARFGAP1 as GST fusion proteins were bound to beads, and then incubated with purified AP-2 followed by incubation with purified clathrin. Beads were then analyzed by immunoblotted for proteins as indicated. GST fusion proteins were detected by Coomassie staining. d. Rescue of defective Tf uptake induced by the depletion of ARFGAP1. BSC-1 cells that stably expressed shRNA against ARFGAP1 were transfected with different rat forms of ARFGAP1 as indicated. Uptake of biotin-Tf at 10 minutes was then quantified. The mean from three experiments with standard error is shown. Difference among conditions of shRNA, FWW and EDE are insignificant (p>0.05). Difference between this group and all other conditions are significant (p<0.05). e. Rescue of defective COPI transport induced by the depletion of ARFGAP1. BSC-1 cells that stably expressed shRNA against ARFGAP1 were transfected with different rat forms of ARFGAP1 as indicated. The redistribution of VSVG-KDELR from the Golgi to the ER at 30 minutes was then quantified. The mean from three experiments with standard error is shown. Difference among conditions of Wt, FWW and EDE are insignificant (p>0.05). Difference between this group and conditions of shRNA and 1–400 are significant (p<0.05).
Figure 4. Surveying transport pathways affected by the depletion of ARFGAP1
a. Surface level of TfR is not affected by ARFGAP1 depletion. An antibody that recognizes the extracellular domain of TfR was bound to BSC-1 cells, followed by quantitation. The mean from three experiments with standard error is shown. Difference between the two conditions is insignificant (p>0.05). b. TfR recycling is reduced by siRNA against ARFGAP1. BSC-1 cells were treated with siRNA against ARFGAP1, and then the level of internal biotin-Tf at time points as indicated was quantified. The mean from three experiments with standard error is shown. Difference between the two conditions (except time = 0) is significant (p<0.05). c. Rescue of defective TfR recycling induced by the depletion of ARFGAP1. BSC-1 cells that stably expressed shRNA against ARFGAP1 were transfected with different forms of rat ARFGAP1 as indicated. The level of internal biotin-Tf that remained was then quantified. The mean with standard error from three experiments is shown. Difference between shRNA and all other conditions is significant (p<0.05). d. Depletion of ARFGAP1 inhibits transport from the ER to the TGN. VSVG-ts045 was transfected into BSC-1 cells, followed by quantitation of its colocalization with a TGN marker (TGN46). The mean from three experiments with standard error is shown. Difference at all time points is significant (p<0.05). e. Depletion of ARFGAP1 does not affect transport from the ER to the cis-side of the Golgi. VSVG-ts045 was transfected into BSC-1 cells, followed by quantitation of its colocalization with a cis-Golgi marker (giantin). The mean from three experiments with standard error is shown. Difference at all time points is insignificant (p>0.05). f. Depletion of ARFGAP1 does not affect transport from the TGN to the PM. VSVG-ts045 was transfected into BSC-1 cells, and then accumulated at the TGN. Cells were then shifted from 20°C to 32°C to allow transport to the plasma membrane (PM). Arrival of VSVG at the PM was detected through colocalization with fluorescence-conjugated CTB (which was bound to the cell surface). The mean from three experiments with standard error is shown. Difference at all time points is insignificant (p>0.05).
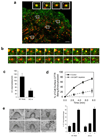
Figure 5. ARFGAP1 affects coated pits formation
a. Colocalization of ARFGAP1 with clathrin in coated pits. BSC-1 cells transfected with GFP-tagged ARFGAP1 and mCherry-tagged clathrin light chain (CLC) were examined by TIR-FM. The merged view shows colocalization of the two proteins; bar, 2 um. Insets highlight examples of coated pits found to have both ARFGAP1 (green) and clathrin (red). b. Dynamic association of ARFGAP1 with clathrin in coated pits. BSC-1 cells transfected with GFP-ARFGAP1 and mCherry-CLC and then examined by TIR-FM with live-imaging. Two examples are shown, with images captured every 3 seconds; bar, 1 µm. c. Depletion of ARFGAP1 reduces the level of coated pits. HeLa cells were treated with siRNA conditions as indicated. All forms of clathrin coated (CC) intermediates were counted and then divided by the length of the plasma membrane. Seven cells were randomly selected from each condition to obtain the mean with standard deviation. Difference between two conditions is significant (p<0.05). d. Depletion of ARFGAP1 inhibits the endocytosis of CFTR. CFBE41o- cells expressing WT-CFTR were polarized and then treated with siRNA conditions as indicated. The level of internalized CFTR was then quantified. The mean with standard error from three experiments is shown. Difference between two conditions (except time = 0) is significant (p<0.05). e. Depletion of ARFGAP1 does not induce a particular stage of coated pit formation to accumulate. The different stages of coated pit formation, with representative images for each stage shown (left; bar, 100 nm), were detected by EM, and then quantified. The mean from three experiments with standard deviation is shown (right). Difference between corresponding stages is insignificant (p>0.05).
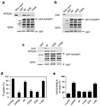
Figure 6. ARFGAP1 and AP-2 interact specifically with surface TfR
a. Endogenous surface TfR interacts with endogenous forms of ARFGAP1 and AP-2 in vivo. Biotin-labeled Tf was bound to the surface of HeLa cells followed by isolation using streptavidin beads, and then immunoblotting for proteins indicated. b. GFP-tagged ARFGAP1 associates with endogenous forms of surface TfR and AP-2. BSC-1 cells were transfected with GFP-tagged ARFGAP1. Cells were then bound with biotin-labeled Tf, followed by incubation of cell lysate with streptavidin beads, and then immunoblotting for proteins indicated. c. Surface TfR does not associate with ARFGAP-2 or ARFGAP3. HeLa cells were transfected with myc-tagged ARFGAP1, or HA-tagged forms of ARFGAP-2 or ARFGAP3. Cells were then bound with biotin-labeled Tf, and then lysed, followed by incubation of cell lysate with streptavidin beads, and then immunoblotting for proteins as indicated. ARFGAPs were detected through antibodies against the epitope tag. d. A surface form of LDLR does not associate with ARFGAP1. HeLa cells that stably expressed CD8-LDLR were first incubated with anti-CD8 antibody. After this surface binding, cells were lysed and then incubated with protein A beads, followed by immunoblotting for proteins as indicated.
Figure 7. Characterizing the binding of TfR by ARFGAP1 and AP-2
a. Pulldown assays were performed using TfR constructs as indicated for incubation with recombinant ARFGAP1, followed by immunoblotting for ARFGAP1. GST fusion proteins were detected by Coomassie staining. b. Pulldown assays were performed using TfR constructs as indicated for incubation with recombinant ARFGAP1, followed by immunoblotting for ARFGAP1. GST fusion proteins were detected by Coomassie staining. Point mutations within particular truncation constructs are indicated within parentheses. c. Pulldown assays were performed using TfR constructs as indicated for incubation with purified AP-2, followed by immunoblotting for β2-adaptin. GST fusion proteins were detected by Coomassie staining. Point mutations within particular truncation constructs are indicated within parentheses. d. Effects on the association of surface TfR with ARFGAP1 and AP-2 upon mutating sorting signals in TfR. Biotin-labelled Tf was bound to the surface of mutant CHO (TRVb) cells that expressed different full-length TfR forms (either wild-type or different point mutations as indicated), followed by isolation using streptavidin beads, and then immunoblotting for proteins indicated. The “4A” construct contains alanine substitutions at residues 12, 13, 22, and 23 of TfR. e. Association of surface TfR with AP-2 requires ARFGAP1. BSC-1 cells were treated with siRNA against ARFGAP1. Biotin-labeled Tf was bound to the surface of BSC-1 cells, followed by isolation using streptavidin beads, and then immunoblotting for endogenous proteins as indicated. Cell lysates were also directly immunoblotted for proteins in conditions as indicated.
Figure 8. The GAP activity of ARFGAP1 is important for TfR endocytosis
a. The GAP activity of ARFGAP1 is important for TfR endocytosis. BSC-1 cells that stably expressed shRNA against ARFGAP1 were transfected with constructs as indicated. Uptake of biotin-Tf at 10 minutes was then quantified. The mean with standard error from three experiments is shown. Difference between the condition of shRNA and rescue by Wt is significant (p<0.05). Difference between the condition of shRNA and rescue by R50K is insignificant (p>0.05). b. Depletion of ARF6 inhibits Tf uptake. BSC-1 cells were treated with siRNA against ARF6. Uptake of biotin-Tf at 10 minutes was then quantified. The mean with standard error from three experiments is shown. Difference between the two conditions is significant (p<0.05). c. AP-2 enhances GAP activity of ARFGAP1 toward ARF6. The GAP assay was performed using ARF6 as the substrate, and either with (triangles) or without (squares) AP-2. The mean from three experiments with standard error is shown. d. Binding of ARF6 to TfR is activation-dependent. ARF6 was confirmed functionally for its activation using the effector domain of GGA3 in a pulldown experiment (left panel). ARF6 forms were also incubated with GST-TfR in another pulldown experiment (right panel). e. GAP activity of ARFGAP1 optimizes the binding of AP-2 to TfR. GST-TfR was incubated sequentially with ARF6 (containing different nucleotide bound, as indicated), followed by ARFGAP1, and then AP-2. f. Deactivation of ARF6 by ARFGAP1 releases ARF6 from binding to TfR. ARF6 loaded with GTP forms as indicated were incubated with GST-TfR along with ARFGAP1 in a pulldown experiment. g. Activation-dependent binding of ARF6 to surface TfR. BSC-1 cells were transfected with point mutant forms of ARF6 as indicated. Surface TfR was then isolated through biotin-Tf binding to cell surface, followed by incubation with streptavidin beads. Immunoblotting was then performed for proteins as indicated. h. Constitutively activated form of ARF6 (Q67L) reduces the binding of AP-2 to surface TfR. BSC-1 cells were transfected with ARF6-Q67L or mock transfected. Surface TfR was then isolated as described above, followed by immunoblotting for associated proteins as indicated.
Similar articles
- ArfGAP1 activity and COPI vesicle biogenesis.
Beck R, Adolf F, Weimer C, Bruegger B, Wieland FT. Beck R, et al. Traffic. 2009 Mar;10(3):307-15. doi: 10.1111/j.1600-0854.2008.00865.x. Epub 2008 Dec 4. Traffic. 2009. PMID: 19055691 - ArfGAP1 dynamics and its role in COPI coat assembly on Golgi membranes of living cells.
Liu W, Duden R, Phair RD, Lippincott-Schwartz J. Liu W, et al. J Cell Biol. 2005 Mar 28;168(7):1053-63. doi: 10.1083/jcb.200410142. J Cell Biol. 2005. PMID: 15795316 Free PMC article. - Differential roles of ArfGAP1, ArfGAP2, and ArfGAP3 in COPI trafficking.
Weimer C, Beck R, Eckert P, Reckmann I, Moelleken J, Brügger B, Wieland F. Weimer C, et al. J Cell Biol. 2008 Nov 17;183(4):725-35. doi: 10.1083/jcb.200806140. J Cell Biol. 2008. PMID: 19015319 Free PMC article. - ArfGAP1 function in COPI mediated membrane traffic: currently debated models and comparison to other coat-binding ArfGAPs.
Shiba Y, Randazzo PA. Shiba Y, et al. Histol Histopathol. 2012 Sep;27(9):1143-53. doi: 10.14670/HH-27.1143. Histol Histopathol. 2012. PMID: 22806901 Free PMC article. Review. - The long life of an endocytic patch that misses AP-2.
de León N, Valdivieso MH. de León N, et al. Curr Genet. 2016 Nov;62(4):765-770. doi: 10.1007/s00294-016-0605-3. Epub 2016 Apr 28. Curr Genet. 2016. PMID: 27126383 Review.
Cited by
- MTV1 and MTV4 encode plant-specific ENTH and ARF GAP proteins that mediate clathrin-dependent trafficking of vacuolar cargo from the trans-Golgi network.
Sauer M, Delgadillo MO, Zouhar J, Reynolds GD, Pennington JG, Jiang L, Liljegren SJ, Stierhof YD, De Jaeger G, Otegui MS, Bednarek SY, Rojo E. Sauer M, et al. Plant Cell. 2013 Jun;25(6):2217-35. doi: 10.1105/tpc.113.111724. Epub 2013 Jun 14. Plant Cell. 2013. PMID: 23771894 Free PMC article. - Role of ArfGAP1 in COPI vesicle biogenesis.
Hsu VW. Hsu VW. Cell Logist. 2011 Mar;1(2):55-56. doi: 10.4161/cl.1.2.15175. Cell Logist. 2011. PMID: 21686254 Free PMC article. - 2-Hydroxypropyl-β-cyclodextrin reduces retinal cholesterol in wild-type and Cyp27a1-/- Cyp46a1-/- mice with deficiency in the oxysterol production.
El-Darzi N, Mast N, Petrov AM, Pikuleva IA. El-Darzi N, et al. Br J Pharmacol. 2021 Aug;178(16):3220-3234. doi: 10.1111/bph.15209. Epub 2020 Aug 13. Br J Pharmacol. 2021. PMID: 32698250 Free PMC article. - Coordinated regulation of bidirectional COPI transport at the Golgi by CDC42.
Park SY, Yang JS, Schmider AB, Soberman RJ, Hsu VW. Park SY, et al. Nature. 2015 May 28;521(7553):529-32. doi: 10.1038/nature14457. Epub 2015 May 6. Nature. 2015. PMID: 25945738 Free PMC article. - ArfGAP1 inhibits mTORC1 lysosomal localization and activation.
Meng D, Yang Q, Melick CH, Park BC, Hsieh TS, Curukovic A, Jeong MH, Zhang J, James NG, Jewell JL. Meng D, et al. EMBO J. 2021 Jun 15;40(12):e106412. doi: 10.15252/embj.2020106412. Epub 2021 May 14. EMBO J. 2021. PMID: 33988249 Free PMC article.
References
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. - PubMed
- Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. - PubMed
- Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. - PubMed
- McMahon HT, Mills IG. COP and clathrin-coated vesicle budding: different pathways, common approaches. Curr Opin Cell Biol. 2004;16:379–391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM075252/GM/NIGMS NIH HHS/United States
- R01 GM058615-08S1/GM/NIGMS NIH HHS/United States
- GM058615/GM/NIGMS NIH HHS/United States
- U54 AI057159/AI/NIAID NIH HHS/United States
- R01 GM073016/GM/NIGMS NIH HHS/United States
- R01 GM058615/GM/NIGMS NIH HHS/United States
- R01 GM073016-05A1/GM/NIGMS NIH HHS/United States
- R01 GM075252/GM/NIGMS NIH HHS/United States
- DK060065/DK/NIDDK NIH HHS/United States
- R37 GM058615/GM/NIGMS NIH HHS/United States
- TGM11CB5/TI_/Telethon/Italy
- R01 DK060065/DK/NIDDK NIH HHS/United States
- GM073016/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous