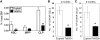Defective hematopoietic stem cell and lymphoid progenitor development in the Ts65Dn mouse model of Down syndrome: potential role of oxidative stress - PubMed (original) (raw)
Defective hematopoietic stem cell and lymphoid progenitor development in the Ts65Dn mouse model of Down syndrome: potential role of oxidative stress
Laureanne Pilar E Lorenzo et al. Antioxid Redox Signal. 2011.
Abstract
Aims: Down Syndrome (DS), a genetic disease caused by a triplication of chromosome 21, is characterized by increased markers of oxidative stress. In addition to cognitive defects, patients with DS also display hematologic disorders and increased incidence of infections and leukemia. Using the Ts65Dn mouse model of DS, the goal of this study was to examine hematopoietic stem and lymphoid progenitor cell function in DS.
Results: Analysis of hematopoietic progenitor populations showed that Ts65Dn mice possessed fewer functional hematopoietic stem cells and a significantly decreased percentage of bone marrow lymphoid progenitors. Increased reactive oxygen species and markers of oxidative stress were detected in hematopoietic stem cell populations and were associated with a loss of quiescence. Bone marrow progenitor populations expressed diminished levels of the IL-7Rα chain, which was associated with decreased proliferation and increased apoptosis. Modulating oxidative stress in vitro suggested that oxidative stress selectively leads to decreased IL-7Rα expression, and inhibits the survival of IL-7Rα-expressing hematopoietic progenitors, potentially linking increased reactive oxygen species and immunopathology.
Innovation: The study results identify a link between oxidative stress and diminished IL-7Rα expression and function. Further, the data suggest that this decrease in IL-7Rα is associated with defective hematopoietic development in Down Syndrome.
Conclusion: The data suggest that hematopoietic stem and lymphoid progenitor cell defects underlie immune dysfunction in DS and that increased oxidative stress and reduced cytokine signaling may alter hematologic development in Ts65Dn mice.
Figures
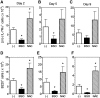
FIG. 1.
Hematopoietic progenitor populations in the bone marrow of Ts65Dn and euploid mice. (A) Hematopoietic progenitor populations as a percentage of nucleated BMC from euploid (open bars) or Ts65Dn (closed bars) mice. Hematopoietic progenitors [long-term reconstituting hematopoietic stem cell (HSC), multipotent progenitor (MPP), and common lymphoid progenitor (CLP) populations] were assessed ex vivo by flow cytometry as defined and described in the Materials and Methods section (_n_=6, *p<0.05; **p<0.005). (B, C) Competitive repopulation assay was performed as described in the Materials and Methods section with 1:1 mixture of BMC from Ts65Dn mice (closed bars) or euploid littermates (open bars) mixed with an equal number of wild-type BM. BMC repopulating ability was assessed at (B) 3 months and (C) 9 months and is expressed as repopulating units per million BMC. By definition 105 competitor BMC produce 1 repopulating unit (_n_=4, *p<0.05). BMC, bone marrow cells.
FIG. 2.
Ts65Dn BMC exhibit signs of oxidative stress. (A) Decreased GSH/GSSG ratio in blood cells from Ts65Dn mice. GSH and GSSG were measured spectrophotometrically in fresh blood samples from euploid and Ts65Dn mice as described in the Materials and Methods section (_n_=5, *p<0.05). (B) 2′-7′-dichlorofluorescin diacetate oxidation was assessed in the indicated bone marrow subpopulations by flow cytometry. These data are expressed as the percent increase in 2′-7′-dichlorofluorescin diacetate fluorescence normalized to uptake of fluorescein diacetate (_n_=4, *p<0.05). (C) Intracellular GSH levels were measured in the indicated bone marrow subsets from euploid (open bars) and Ts65Dn (closed bars) mice using monochlorobimane as described in the Materials and Methods section (_n_=5, *p<0.05). (D) Formation of 3-nitrotyrosine (3-NT) was detected in the indicated bone marrow subsets from euploid (open bars) or Ts65Dn (closed bars) mice by intracellular staining and FACS analysis as described in the Materials and Methods section (_n_=5, *p<0.05). (E) Formation of protein carbonyls (PC) were detected in the indicated bone marrow subsets from euploid (open bars) or Ts65Dn (closed bars) mice by intracellular staining and FACS analysis as described in the Materials and Methods section (_n_=5, *p<0.05). GSH, glutathione; GSSG, oxidized glutathione.
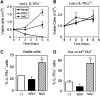
FIG. 3.
Ts65Dn BMC exhibit signs of loss of quiescence. (A) Representative FACS plots of HSC cell cycle analysis using Ki-67 and 7-AAD in Euploid and Ts65Dn mice. (B) Intracellular staining for the Ki-67 antigen was analyzed by flow cytometry as described in the Materials and Methods section. The percentage of cells that are Ki-67(−) were analyzed in total BMC (live gate), lineage-negative (Lin(−)), (Lin−), Sca-1+, c-Kit+ (LSK) population, and the Lin(−), LSK Flk2- (HSC) population. (_n_=5, *p<0.05). (C) HSC cell cycle analysis by analysis of Ki-67 expression and 7-AAD incorporation showing the percentage of cells in G0, G1, and G2+M phases. (_n_=4, *p<0.05). 7-AAD, 7-Amino-Actinomycin D.
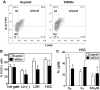
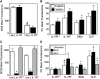
FIG. 4.
Downregulation of IL-7Rα and Flk2 expression and function in the bone marrow of Ts65Dn mice. (A, B) IL-7Rα expression in selected bone marrow sub-populations was assessed by flow cytometry. (A) Representative FACS plots of IL-7Rα expression in LSK or the Lin(−), SKlo, Flk2+ populations from euploid (solid line) or Ts65Dn (dashed lines) mice. (B) Analysis of the percent cells that are IL-7Rα+ in total BMC (live gate), Lin(−), Lin(−), Sca-1lo,c-Kitlo (SKlo) population, and the Lin(−), SKlo, Flk2+ (Flk2+) population. (_n_=6, *p<0.05, **p<0.01). (C, D) Flk2 expression in selected bone marrow sub-populations was assessed by flow cytometry. (C) Representative FACS plots of Flk2 expression in LSK cells from euploid (solid line) or Ts65Dn (dashed lines) mice. Marker indicates Flk2-bright population. (D) Analysis of the percent cells that are Flk2+ in total BMC (live gate), Lin(−), Lin(−), Sca-1lo,c-Kitlo (SKlo) population, and the LSK population (_n_=6, *p<0.05, **p<0.01). (E) Colony formation of Ts65Dn and euploid pre-B cells. Total BMC were cultured in methylcellulose media containing IL-7 and enumerated after 7 days. CFU pre-B: pre-B cell colony forming unit (_n_=4, *p<0.05). (F) Cell cycle analysis by Ki-67 expression and 7-AAD incorporation of Lin(−) IL-7Rα+ (Lin- IL-7Rα+) lymphoid progenitor cells from Ts65Dn mice (closed bars) or euploid littermates (open bars) (_n_=4, *p<0.05). (G) Apoptotic cells were detected by AnnexinV binding in selected bone marrow sub-populations from Ts65Dn mice (closed bars) or euploid littermates (open bars). These data are expressed as the percentage of cells in each population that are AnnexinV+ (_n_=6, *p<0.05).
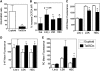
FIG. 5.
Redox balance affects IL-7Rα expression and B-cell generation in vitro. IL-7Rα (A–C) and B220 (D–F) expression was assessed by flow cytometry in lymphoid progenitor cultures. Lineage-depleted hematopoietic progenitors were cultured in vitro in lymphoid-promoting media as described in the Materials and Methods section. The pro-oxidant BSO (1 m_M_) and antioxidant NAC (1 m_M_) were added to selected cultures. IL-7Rα expression was assessed in Lin(−) cells (Lin(−) IL-7Rα+) on day 2 (A), day 5 (B), and day 9 (C). B220 expression was assessed on day 2 (D), day 5 (E), and day 9 (F). The data are expressed as the number of cells in each sub-population (_n_=3, *p<0.05). BSO, buthionine sulfoximine; NAC, acetylcysteine.
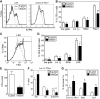
FIG. 6.
Pro/anti-oxidant treatment specifically affects the viability of IL-7Rα-expressing cells. FACS-sorted Lin(−), IL-7Rα+ (A) and Lin(−), IL-7Rα (−) (B) hematopoietic progenitors were cultured in vitro in lymphoid-promoting media. The pro-oxidant BSO (0.1 m_M_) and antioxidant NAC (1 m_M_) were added to selected cultures and the number of viable cells in each culture condition was assessed using 7-AAD exclusion by flow cytometry on days 2 and 5 (_n_=2, *p<0.05). (C, D) The percentage of IL-7Rα+ cells was assessed in FACS-sorted Lin(−), IL-7Rα+ hematopoietic progenitors in the total viable (C) and Sca-1lo c-Kitlo Flk2+ (D) sub-populations by flow cytometry (_n_=3, *p<0.05).
FIG. 7.
Oxidative stress in IL-7Rα+ BMC both ex vivo and in vitro . (A, B) BMC from euploid (open bars) and Ts65Dn (closed bars) mice were analyzed ex vivo for (A) intracellular GSH in Lin(−), IL-7Rα(−) or Lin(−), IL-7Rα+ cells or (B) PCs in Lin(−), IL-7Rα(−) (IL-7Rα(−)), Lin(−), IL-7Rα+ (IL-7Rα+), Lin(−), Sca-1lo,c-Kitlo (SKlo), and the Lin(−), SKlo, Flk2+ IL-7Rα+ (CLP) populations as in Figure 2. (C, D) BMC from wild-type mice were cultured in vitro in lymphoid-promoting media in the absence of any addition (open bars), or in the presence of 0.1 m_M_ BSO (solid bars) or 1 m_M_ NAC (hatched bars) for 2 days as in Figure 5. Cells were analyzed for (C) intracellular GSH or (D) PCs in the indicated subsets as defined in A & B (_n_=5, *significantly different from euploid or untreated controls, p<0.05; #significantly different from IL-7R(−) samples, p<0.05).
Similar articles
- Defective thymic progenitor development and mature T-cell responses in a mouse model for Down syndrome.
Lorenzo LP, Shatynski KE, Clark S, Yarowsky PJ, Williams MS. Lorenzo LP, et al. Immunology. 2013 Aug;139(4):447-58. doi: 10.1111/imm.12092. Immunology. 2013. PMID: 23432468 Free PMC article. - Hematopoietic Stem Cells from Ts65Dn Mice Are Deficient in the Repair of DNA Double-Strand Breaks.
Wang Y, Chang J, Shao L, Feng W, Luo Y, Chow M, Du W, Meng A, Zhou D. Wang Y, et al. Radiat Res. 2016 Jun;185(6):630-7. doi: 10.1667/RR14407.1. Epub 2016 May 31. Radiat Res. 2016. PMID: 27243896 Free PMC article. - Highly penetrant myeloproliferative disease in the Ts65Dn mouse model of Down syndrome.
Kirsammer G, Jilani S, Liu H, Davis E, Gurbuxani S, Le Beau MM, Crispino JD. Kirsammer G, et al. Blood. 2008 Jan 15;111(2):767-75. doi: 10.1182/blood-2007-04-085670. Epub 2007 Sep 27. Blood. 2008. PMID: 17901249 Free PMC article. - Developmental instability of the cerebellum and its relevance to Down syndrome.
Shapiro BL. Shapiro BL. J Neural Transm Suppl. 2001;(61):11-34. doi: 10.1007/978-3-7091-6262-0_2. J Neural Transm Suppl. 2001. PMID: 11771737 Review. - Oxidative stress in normal hematopoietic stem cells and leukemia.
Samimi A, Kalantari H, Lorestani MZ, Shirzad R, Saki N. Samimi A, et al. APMIS. 2018 Apr;126(4):284-294. doi: 10.1111/apm.12822. APMIS. 2018. PMID: 29575200 Review.
Cited by
- Tumorigenesis in Down's syndrome: big lessons from a small chromosome.
Nižetić D, Groet J. Nižetić D, et al. Nat Rev Cancer. 2012 Oct;12(10):721-32. doi: 10.1038/nrc3355. Epub 2012 Sep 21. Nat Rev Cancer. 2012. PMID: 22996602 - Usp16: key controller of stem cells in Down syndrome.
Xu JC, Dawson VL, Dawson TM. Xu JC, et al. EMBO J. 2013 Oct 30;32(21):2788-9. doi: 10.1038/emboj.2013.220. Epub 2013 Sep 27. EMBO J. 2013. PMID: 24076652 Free PMC article. - Defective thymic progenitor development and mature T-cell responses in a mouse model for Down syndrome.
Lorenzo LP, Shatynski KE, Clark S, Yarowsky PJ, Williams MS. Lorenzo LP, et al. Immunology. 2013 Aug;139(4):447-58. doi: 10.1111/imm.12092. Immunology. 2013. PMID: 23432468 Free PMC article. - Gain of chromosome 21 in hematological malignancies: lessons from studying leukemia in children with Down syndrome.
Laurent AP, Kotecha RS, Malinge S. Laurent AP, et al. Leukemia. 2020 Aug;34(8):1984-1999. doi: 10.1038/s41375-020-0854-5. Epub 2020 May 20. Leukemia. 2020. PMID: 32433508 Free PMC article. Review. - Premature aging in aneuploid yeast is caused in part by aneuploidy-induced defects in Ribosome Quality Control.
Escalante LE, Hose J, Howe H, Paulsen N, Place M, Gasch AP. Escalante LE, et al. bioRxiv [Preprint]. 2024 Jun 23:2024.06.22.600216. doi: 10.1101/2024.06.22.600216. bioRxiv. 2024. PMID: 38948718 Free PMC article. Preprint.
References
- Akashi K. Traver D. Miyamoto T. Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. - PubMed
- Allman D. Sambandam A. Kim S. Miller JP. Pagan A. Well D. Meraz A. Bhandoola A. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4:168–174. - PubMed
- Bensaad K. Vousden KH. Savior and slayer: the two faces of p53. Nat Med. 2005;11:1278–1279. - PubMed
- Cheng T. Rodrigues N. Shen H. Yang Y. Dombkowski D. Sykes M. Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous