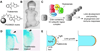Chemical 'Jekyll and Hyde's: small-molecule inhibitors of developmental signaling pathways - PubMed (original) (raw)
Review
. 2011 Aug;40(8):4318-31.
doi: 10.1039/c1cs15019g. Epub 2011 Apr 19.
Affiliations
- PMID: 21505654
- PMCID: PMC3137710
- DOI: 10.1039/c1cs15019g
Review
Chemical 'Jekyll and Hyde's: small-molecule inhibitors of developmental signaling pathways
Tomoyo Sakata et al. Chem Soc Rev. 2011 Aug.
Abstract
Small molecules that perturb developmental signaling pathways can have devastating effects on embryonic patterning, as evidenced by the chemically induced onset of cyclopic lambs and children with severely shortened limbs during the 1950s. Recent studies, however, have revealed critical roles for these pathways in human disorders and diseases, spurring the re-examination of these compounds as new targeted therapies. In this tutorial review, we describe four case studies of teratogenic compounds, including inhibitors of the Hedgehog (Hh), Wnt, and bone morphogenetic protein (BMP) pathways. We discuss how these teratogens were discovered, their mechanisms of action, their utility as molecular probes, and their potential as therapeutic agents. We also consider current challenges in the field and possible directions for future research.
This journal is © The Royal Society of Chemistry 2011
Figures
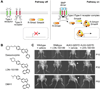
Figure 1. Pharmacological inhibition of Hh signaling
(A) Cyclopic lamb resulting from in utero exposure to the natural product cyclopamine. Photo courtesy of the USDA-Agricultural Research Service, Poisonous Plant Research Lab, Logan, Utah. (B) Chemical structures of cyclopamine and synthetic derivatives used to identify its cellular target. Individual ring systems within the cyclopamine skeleton are labeled A–F. (C) Schematic representation of the Hh pathway, showing the trafficking of signaling proteins through the microtubule-containing primary cilium and nucleus (dashed box). Key phosphorylation events are indicated by the black circles and the putative Gli activation step is depicted by the red diamond. (D) Chemical structures of selected Smo antagonists currently being evaluated in human clinical trials. (E) Response and relapse of metastatic medulloblastoma (dark signals) to GDC-0449 therapy. Reprinted with permission from the Massachusetts Medical Society (Ref. , copyright 2009).
Figure 2. Pharmacological inhibition of Wnt signaling
(A) Schematic representation of the Wnt pathway, with key phosphorylation events indicated by the black circles and the nucleus depicted by the dashed box. (B) Chemical structures of the Wnt pathway inhibitors IWR-1, IWR-3, and XAV939.
Figure 3. Pharmacological inhibition of BMP signaling
(A) Schematic representation of the BMP pathway, with key phosphorylation events indicated by the black circles and the nucleus depicted by the dashed box. (B) Chemical structures of dorsomorphin and its chemical analogs LDN-193189 and DMH1. (C) Reduction of heterotopic ossification by LDN-193189 in a mouse model of fibrodysplasia ossificans progressiva. X-ray images of mice with ectopic expression of ALK2-Q207D in their left hindlegs are shown, with soft tissue calcification resulting in animals treated with vehicle alone (arrowheads). Adapted by permission from Macmillian Publishers Ltd: Nature Medicine (Ref. , copyright 2008).
Figure 4. Thalidomide and its mechanism of action
(A) Chemical structures of thalidomide and an affinity matrix conjugated to its carboxyl derivative FR259625. (B) Limb deformities caused by in utero exposure to thalidomide. Reprinted with permission from the British Medical Journal Publishing Group, Ltd. (Ref. , copyright 1992). (C) Inhibition of Crbn-dependent ubiquitination of a putative protein that is required limb development and possibly angiogenesis and immune responses. (D) Pectoral defects (arrowheads) in thalidomide-treated zebrafish embryos. Adapted with permission from the American Association for the Advancement of Science (Ref. , copyright 2010). (E) Schematic representation of the Fgf8/Fgf10 feedback loop that promotes limb outgrowth, with the Fgf8-expressing apical epidermal ridge depicted in orange and the underlying Fgf10-expressing mesenchyme depicted in blue.
Similar articles
- From teratogens to potential therapeutics: natural inhibitors of the Hedgehog signaling network come of age.
Hovhannisyan A, Matz M, Gebhardt R. Hovhannisyan A, et al. Planta Med. 2009 Oct;75(13):1371-80. doi: 10.1055/s-0029-1185979. Epub 2009 Jul 28. Planta Med. 2009. PMID: 19639534 Review. - A looking glass perspective: thalidomide and cyclopamine.
Gaffield W, Incardona JP, Kapur RP, Roelink H. Gaffield W, et al. Cell Mol Biol (Noisy-le-grand). 1999 Jul;45(5):579-88. Cell Mol Biol (Noisy-le-grand). 1999. PMID: 10512190 Review. - I only have eye for ewe: the discovery of cyclopamine and development of Hedgehog pathway-targeting drugs.
Chen JK. Chen JK. Nat Prod Rep. 2016 May 4;33(5):595-601. doi: 10.1039/c5np00153f. Nat Prod Rep. 2016. PMID: 26787175 Free PMC article. Review. - Applications of small molecule BMP inhibitors in physiology and disease.
Hong CC, Yu PB. Hong CC, et al. Cytokine Growth Factor Rev. 2009 Oct-Dec;20(5-6):409-18. doi: 10.1016/j.cytogfr.2009.10.021. Epub 2009 Nov 14. Cytokine Growth Factor Rev. 2009. PMID: 19914855 Free PMC article. Review. - Small-molecule modulators of Hh and Wnt signaling pathways.
Kiselyov AS, Tkachenko SE, Balakin KV, Ivachtchenko AV. Kiselyov AS, et al. Expert Opin Ther Targets. 2007 Aug;11(8):1087-101. doi: 10.1517/14728222.11.8.1087. Expert Opin Ther Targets. 2007. PMID: 17665980 Review.
Cited by
- Safely targeting cancer stem cells via selective catenin coactivator antagonism.
Lenz HJ, Kahn M. Lenz HJ, et al. Cancer Sci. 2014 Sep;105(9):1087-92. doi: 10.1111/cas.12471. Epub 2014 Sep 6. Cancer Sci. 2014. PMID: 24975284 Free PMC article. Review. - Bicyclic β-Sheet Mimetics that Target the Transcriptional Coactivator β-Catenin and Inhibit Wnt Signaling.
Wendt M, Bellavita R, Gerber A, Efrém NL, van Ramshorst T, Pearce NM, Davey PRJ, Everard I, Vazquez-Chantada M, Chiarparin E, Grieco P, Hennig S, Grossmann TN. Wendt M, et al. Angew Chem Int Ed Engl. 2021 Jun 14;60(25):13937-13944. doi: 10.1002/anie.202102082. Epub 2021 May 5. Angew Chem Int Ed Engl. 2021. PMID: 33783110 Free PMC article. - Novel Vein Patterns in Arabidopsis Induced by Small Molecules.
Carland F, Defries A, Cutler S, Nelson T. Carland F, et al. Plant Physiol. 2016 Jan;170(1):338-53. doi: 10.1104/pp.15.01540. Epub 2015 Nov 16. Plant Physiol. 2016. PMID: 26574596 Free PMC article. - Precision medicine for advanced prostate cancer.
Mullane SA, Van Allen EM. Mullane SA, et al. Curr Opin Urol. 2016 May;26(3):231-9. doi: 10.1097/MOU.0000000000000278. Curr Opin Urol. 2016. PMID: 26909474 Free PMC article. Review. - The Smad Dependent TGF-β and BMP Signaling Pathway in Bone Remodeling and Therapies.
Zou ML, Chen ZH, Teng YY, Liu SY, Jia Y, Zhang KW, Sun ZL, Wu JJ, Yuan ZD, Feng Y, Li X, Xu RS, Yuan FL. Zou ML, et al. Front Mol Biosci. 2021 May 5;8:593310. doi: 10.3389/fmolb.2021.593310. eCollection 2021. Front Mol Biosci. 2021. PMID: 34026818 Free PMC article. Review.
References
- Speirs AL. Lancet. 1962;1:303–305. - PubMed
- Binns W, Thacker EJ, James LF, Huffman WT. J. Am. Vet. Med. Assoc. 1959;134:180–183. - PubMed
- Binns W, James LF, Shupe JL, Everett G. Am. J. Vet. Res. 1963;24:1164–1175. - PubMed
- Keeler RF, Binns W. Can. J. Biochem. 1966;44:819–828. - PubMed
- Keeler RF, Binns W. Can. J. Biochem. 1966;44:829–838. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources