Horizontal gene transfers with or without cell fusions in all categories of the living matter - PubMed (original) (raw)
Review
Horizontal gene transfers with or without cell fusions in all categories of the living matter
Joseph G Sinkovics. Adv Exp Med Biol. 2011.
Abstract
This article reviews the history of widespread exchanges of genetic segments initiated over 3 billion years ago, to be part of their life style, by sphero-protoplastic cells, the ancestors of archaea, prokaryota, and eukaryota. These primordial cells shared a hostile anaerobic and overheated environment and competed for survival. "Coexist with, or subdue and conquer, expropriate its most useful possessions, or symbiose with it, your competitor" remain cellular life's basic rules. This author emphasizes the role of viruses, both in mediating cell fusions, such as the formation of the first eukaryotic cell(s) from a united crenarchaeon and prokaryota, and the transfer of host cell genes integrated into viral (phages) genomes. After rising above the Darwinian threshold, rigid rules of speciation and vertical inheritance in the three domains of life were established, but horizontal gene transfers with or without cell fusions were never abolished. The author proves with extensive, yet highly selective documentation, that not only unicellular microorganisms, but the most complex multicellular entities of the highest ranks resort to, and practice, cell fusions, and donate and accept horizontally (laterally) transferred genes. Cell fusions and horizontally exchanged genetic materials remain the fundamental attributes and inherent characteristics of the living matter, whether occurring accidentally or sought after intentionally. These events occur to cells stagnating for some 3 milliard years at a lower yet amazingly sophisticated level of evolution, and to cells achieving the highest degree of differentiation, and thus functioning in dependence on the support of a most advanced multicellular host, like those of the human brain. No living cell is completely exempt from gene drains or gene insertions.
Figures
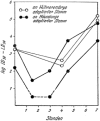
Fig. 2.1
Graph from “Die Grundlagen der Virusforschung (1956)” showing the disappearance (“eclipse”) of all detectable viral activity (“die Dauer des Vermehrungszyklus die Infektionsfähigkeit verliert”) after the inoculation of influenza A virus into the allantois cavity of a chicken embryo, or into a mouse lung, and the reappearance of viral structural proteins and then mature extracellular infectious virions. The work started in 1950 and was published by Sinkovics and Molnár in 1954 [, cited in 180]. Permission to re-publish is from Akadémiai Kiadó, Budapest
Fig. 2.2 (a)
Human sarcoma cells attacked and lysed by autologous lymphocytes. The compact small round cells are immune T cells. It appears as if the lymphocytes injected “cytolysins” into the cytoplasms. The large granular lymphoid cells in 10.21 are NK cells. (b) In 3.8, human sarcoma cells withstand attack by a mixed population of lymphocytes; some lymphocytes die apoptotic death (arrows) next to the attacked tumor cell. From the Section of Clinical Tumor Virology & Immunology, M. D. Anderson Hospital, Houston, TX, in the early 1970s [213]. Permission to re-publish is from Schenk Buchverlag, Passau and Budapest
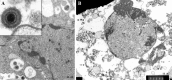
Fig. 2.3 (a)
Classical Mediterranean Kaposi’s sarcoma cells from the pre-AIDS era, in the early 1970s. Herpesvirus particles are those of HHV-8 (KSHV) (not known at that time). (b) Unidentified budding retrovirus particles (different in morphology from HIV-1) are those of an activated endogenous retrovirus (four arrows) in the disintegrating cytoplasm of a Kaposi’s sarcoma cell. Next to the nucleus (single arrow) a mature herpesvirus-like particle is present (HHV-8, unidentified). The cell nucleus contains immature herpes-like virus particles. From the Department of Pathology (chief, Prof. Ferenc Györkey†), Veterans’ Administration Hospital Medical Center, Houston, TX [213]. Permission to re-publish is from Schenk Buchverlag, Passau and Budapest
Fig. 2.4 (a,b)
Spontaneous cell fusions in murine lymphoma observed in the mid-1960s at the Section of Clinical Tumor Virology & Immunology, M. D. Anderson Hospital, Houston, TX. The retrovirally transformed lymphoma cells showed budding retroviral particles (retroviral envelopes) in their cell membrane. The immune B (plasma) cells produced immunoglobulins specifically reacting with structural proteins of the virus particles budding from the lymphoma cells. The immunoglobulins neutralized infectious retrovirus in a spleen focus assay. The lymphoma cells and the immune plasma cells adhered to one another and fused. The fused products were tetra- or polyploid, grew in suspension cultures for over 10 years (a) and in the peritoneal cavity of mice, secreted the specific immunoglobulin, and were attacked by macrophages inducing the “starry sky” phenomenon (b). [767] The native spontaneous cell fusion event was duplicated in the peritoneal cavity of mice. Co-inoculated mixtures of lymphoma cells and immune plasma cells fused and produced bi-nucleated cells (b) [–772]. In his first report on these fused cells, the author wrote in Lancet: “Tetraploid immunoresistant lymphoma cells in the mouse emerge by fusion of the diploid virus-producing lymphoma cell with a plasma cell producing virus-specific globulins. The resulting tetraploid cell will retain malignant growth potential and the genetically determined committedness of both parent cells – to produce leukemia virus, as coded for by the viral genome within the neoplastic cell, and to synthesize virus-specific globulins, as coded for by the genome of the plasma cell” [213, 765]. The USA National Cancer Institute replied in the mid-1970s to the author’s grant applications: “approved without funding, due to low priority.” The circumstances of this work were investigated, validated and credited for priority by Professor Milton Wainwright, University of Sheffield, Sheffield, England [819, 820]. Permission to re-publish is from Schenk Buchverlag, Passau and Budapest
Similar articles
- Horizontal gene transfers and cell fusions in microbiology, immunology and oncology (Review).
Sinkovics JG. Sinkovics JG. Int J Oncol. 2009 Sep;35(3):441-65. doi: 10.3892/ijo_00000357. Int J Oncol. 2009. PMID: 19639166 Review. - The place of viruses in the "tree of life".
Sinkovics JG. Sinkovics JG. Acta Microbiol Immunol Hung. 2001;48(1):115-27. doi: 10.1556/amicr.48.2001.1.11. Acta Microbiol Immunol Hung. 2001. PMID: 11233696 - Evolution of gene fusions: horizontal transfer versus independent events.
Yanai I, Wolf YI, Koonin EV. Yanai I, et al. Genome Biol. 2002;3(5):research0024. doi: 10.1186/gb-2002-3-5-research0024. Epub 2002 Apr 26. Genome Biol. 2002. PMID: 12049665 Free PMC article. - Mimivirus reveals Mre11/Rad50 fusion proteins with a sporadic distribution in eukaryotes, bacteria, viruses and plasmids.
Yoshida T, Claverie JM, Ogata H. Yoshida T, et al. Virol J. 2011 Sep 7;8:427. doi: 10.1186/1743-422X-8-427. Virol J. 2011. PMID: 21899737 Free PMC article. - Pathogenicity islands and phages in Vibrio cholerae evolution.
Faruque SM, Mekalanos JJ. Faruque SM, et al. Trends Microbiol. 2003 Nov;11(11):505-10. doi: 10.1016/j.tim.2003.09.003. Trends Microbiol. 2003. PMID: 14607067 Review.
Cited by
- Hybrid Formation and Fusion of Cancer Cells In Vitro and In Vivo.
Hass R, von der Ohe J, Dittmar T. Hass R, et al. Cancers (Basel). 2021 Sep 6;13(17):4496. doi: 10.3390/cancers13174496. Cancers (Basel). 2021. PMID: 34503305 Free PMC article. Review. - The cnidarian origin of the proto-oncogenes NF-κB/STAT and WNT-like oncogenic pathway drives the ctenophores (Review).
Sinkovics JG. Sinkovics JG. Int J Oncol. 2015 Oct;47(4):1211-29. doi: 10.3892/ijo.2015.3102. Epub 2015 Jul 23. Int J Oncol. 2015. PMID: 26239915 Free PMC article. Review. - Quantification of cell fusion events human breast cancer cells and breast epithelial cells using a Cre-LoxP-based double fluorescence reporter system.
Mohr M, Tosun S, Arnold WH, Edenhofer F, Zänker KS, Dittmar T. Mohr M, et al. Cell Mol Life Sci. 2015 Oct;72(19):3769-82. doi: 10.1007/s00018-015-1910-6. Epub 2015 Apr 22. Cell Mol Life Sci. 2015. PMID: 25900663 Free PMC article. - The cell survival pathways of the primordial RNA-DNA complex remain conserved in the extant genomes and may function as proto-oncogenes.
Sinkovics JG. Sinkovics JG. Eur J Microbiol Immunol (Bp). 2015 Mar;5(1):25-43. doi: 10.1556/EUJMI-D-14-00034. Epub 2015 Mar 26. Eur J Microbiol Immunol (Bp). 2015. PMID: 25883792 Free PMC article. Review. - Development of a lentivirus vector-based assay for non-destructive monitoring of cell fusion activity.
Neshati Z, Liu J, Zhou G, Schalij MJ, de Vries AA. Neshati Z, et al. PLoS One. 2014 Jul 16;9(7):e102433. doi: 10.1371/journal.pone.0102433. eCollection 2014. PLoS One. 2014. PMID: 25028973 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources