Balancing forces: architectural control of mechanotransduction - PubMed (original) (raw)
Review
Balancing forces: architectural control of mechanotransduction
Christopher C DuFort et al. Nat Rev Mol Cell Biol. 2011 May.
Abstract
All cells exist within the context of a three-dimensional microenvironment in which they are exposed to mechanical and physical cues. These cues can be disrupted through perturbations to mechanotransduction, from the nanoscale-level to the tissue-level, which compromises tensional homeostasis to promote pathologies such as cardiovascular disease and cancer. The mechanisms of such perturbations suggest that a complex interplay exists between the extracellular microenvironment and cellular function. Furthermore, sustained disruptions in tensional homeostasis can be caused by alterations in the extracellular matrix, allowing it to serve as a mechanically based memory-storage device that can perpetuate a disease or restore normal tissue behaviour.
Conflict of interest statement
Competing interests statement
The authors declare no competing financial interests.
Figures
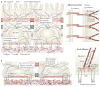
Figure 1. The mechanical network
a | Tissues are mechanically integrated structures, the physical behaviour of which is defined by interconnected networks of cell–cell junctions, cell–matrix adhesions, intracellular filament networks (of actin, microtubules and intermediate filaments) and the extracellular matrix (ECM). Embedded throughout the network are mechanotransducing machines that convert mechanical stimuli into biochemical signals. This process, termed mechanotransduction, enables cells and tissues to sense and respond to their physical surroundings. The ECM controls network connectivity and tension on the network, thereby regulating sites of mechanotransduction. b | Cell–matrix adhesion complexes containing integrins can also directly sense the physical properties of the ECM. These complexes contain specialized protein sensors, including talin, p130Cas (also known as BCAR1), and integrins themselves, that undergo force-dependent conformational changes to elicit downstream signalling responses. The physical properties of the ECM are determined by its composition, the organization of its components, and their degree of intramolecular and intermolecular crosslinking. Interactions between the cell and ECM are dynamic, interwoven and reciprocal. Transcellular tension transmitted across adherens junctions affects ECM remodelling, which in turn regulates cell–matrix and cell–cell adhesions. Increased ECM stiffness owing to remodelling can result in changes in cell and nuclear shape, chromatin organization, assembly of cell–matrix adhesions (called focal adhesions), formation of actin stress fibres, destabilization of cell cell adhesions, and changes in microtubule dynamics. FAK, focal adhesion kinase.
Figure 2. Spatio-mechanical regulation of signalling pathways
Evidence suggests that the mechanically induced spatial organization and clustering of cell surface receptors by the physical properties of materials, including the extracellular matrix (ECM), cell membrane, and cytoskeleton, can regulate a number of signal transduction pathways,,. Juxtacrine signalling is one example in which physical contact between two cells is required for signalling, owing to the receptor (for example, ephrin A receptor 2 (EPHA2)) and ligand (for example, ephrin A1) pairs being presented on apposed cell membranes. a,b | To demonstrate how the mechanics of the microenvironment regulate EPHA2 ephrin A1 signalling in mammary epithelial cells, nanofabricated substrates, consisting of 10 nm high and 100 nm thick chromium lines arranged in a grid pattern (unrestricted (no pattern), 3 μm pitch and 1 μm pitch barriers), were used as a support for a membrane functionalized with laterally mobile (arrows) and fluorescently labelled ephrin A1 ligand, as schematically depicted (a). Representative bright field (BF) and epifluorescence images are also shown (b). When unimpeded, EPHA2 forms clusters with filamentous actin (F-actin) localized at the cell periphery, and is transported radially towards the centre of the cell upon ligand binding in an actomyosin-dependent mechanism (b, no pattern). However, the grid pattern creates diffusion barriers to receptor–ligand complexes, impeding their movement and resulting in their accumulation at barrier boundaries (b, ephrin A1: 3 μm and 1 μm pitch). Distinct cluster patterns yielded different signalling responses and changes in cytoskeletal morphology, which demonstrated a functional link between mechanical environment, receptor organization, signal activation and cell phenotype (b, F actin). Images are modified, with permission, from REF. (2010) AAAS.
Figure 3. The extracellular matrix and tensional homeostasis in development
In tissue and organ morphogenesis, mechanical forces generated by morphogenetic movements play an important part in gene expression by activating developmental biochemical signalling cascades. Embryogenesis provides an example of a multistage process that requires mechanical force, the subsequent localization of proteins, and biochemical signalling to work together. In this process, the cytoskeleton of the cells themselves has a key role in generating the contractile forces required for invagination, gastrulation, proliferation and differentiation,. During the onset of gastrulation, an early phase of embryonic development in Drosophila melanogaster, actin filaments are contracted by non-muscle myosins. Compressive forces result in the ectopic expression of the transcription factor Twist (TWI),, which then directs significant changes in the shape of the developing embryo. When compressive forces (depicted by arrows in the schematic) are disrupted through the laser-ablation of dorsal cells (red region), there is a corresponding reduction in the level of mechanically induced TWI expression in the stomodeal primordium (white arrows) (ablated). Cell nuclei are visualized with a nuclear localization signal-tagged green fluorescent protein (NLS GFP) and immunofluorescence shows the distribution of TWI. Upon gentle compression of the stomodeal cells using a needle, TWI expression is restored in a mechanically induced mechanism (ablated, indented). Figure is modified, with permission, from REF. (2008) Elsevier.
Figure 4. Sustaining mechanotransduction
A | Mechanotransduction occurs on very fast timescales. Actomyosin cables contract at a rate of ~1 μm s–1 (Aa), and stress waves generated by contractility can rapidly propagate across cytoskeletal networks and structural materials (Ab). Stress on mechanically sensitive proteins (mechanosensors) can induce conformational changes, such as protein unfolding, on the order of milliseconds to seconds for pico Newton-level forces (Ac). During mechanotransduction, these changes in protein conformation activate biochemical signalling networks (Ad), through which signals originating at the cell membrane can travel to the nucleus in tens of seconds. Mechanical signalling thus occurs rapidly on timescales of seconds or less. B | Diseases that are linked to altered mechanical signalling require perturbations to be sustained. This can be achieved by genetically controlling the steady-state levels of mechanotransduction machinery and associated regulators. Alternatively, modifying the tissue architecture might sustain mechanical signalling perturbations. The distribution of forces throughout the tissue, and hence of the local sites of mechanotransduction, depend on cell shape, position and connectivity, as well as the intracellular organization of the cytoskeleton and other structural elements. C | The extracellular matrix (ECM), which largely determines tension in tissues, is remodelled during development and disease, which results in altered patterns of tension that can persist for hours, months or even longer (for example, in scar tissue) (Ca). Remodelling enzymes, including the crosslinking enzyme lysyl oxidase (LOX), alter the mechanical properties and spatial topology of the ECM (Cb). Enzymatic (LOX family; red) and non-enzymatic (glycation-mediated; blue) crosslinking in mature collagen filaments is particularly stable and can persist for decades in healthy adults. However, the enhanced activity of enzymes such as LOX in disease can enhance crosslinking, contribute to tissue stiffening, and alter mechanotransduction for prolonged durations.
Figure 5. Tensional homeostasis in tumour progression
Extracellular matrix (ECM) stiffness has been shown to be a potent regulator of cellular behaviour by affecting growth, survival, motility and differentiation. Tensional homeostasis requires a balance between the forces exerted on cells by the ECM and the reciprocal forces generated by cells themselves. When this balance is disrupted by stiffening the ECM, a number of signalling pathways can be adversely affected. a | The collagen crosslinking enzyme lysyl oxidase (LOX) mediates collagen crosslinking and remodelling during breast tumour progression, which results in ECM stiffening. Second-harmonic generation imaging, a type of nonlinear microscopy, was used to show that the collagen in regions adjacent to epithelial lesions in mouse mammary glands undergoes significant morphological modifications, becoming progressively more linear during tumour progression. This remodelling of the ECM was also found to correlate with increased collagen crosslinking and the amount of the crosslinking enzyme LOX, suggesting an association with ECM stiffness, tissue fibrosis and breast tumorigenesis. b | However, matrix remodelling alone is insufficient to promote tumour invasion. Mammary epithelial cells cultured in three-dimensional gels assembled spherical acini, as shown with immunofluorescence of basally oriented β4 integrin and β catenin localized at cell cell junctions (control). Acini that only had the oncogene ERBB2 activated failed to produce an invasive phenotype (+ ERBB2), as did stiffening by nonspecific ribose crosslinking (+ Ribose). Invasion only occurred when stiffening was accompanied with oncogene activation (+ Ribose, + ERBB2). This suggests that matrix remodelling and oncogene activation synergistically induce metastatic progression and that inhibiting LOX and LOX-like enzymes could reduce tumour metastasis by modifying cell ECM interactions. DAPI, 4′,6-diamidino-2-phenylindole. Image is modified, with permission, from REF. (2009) Elsevier.
Similar articles
- Traction force microscopy for understanding cellular mechanotransduction.
Hur SS, Jeong JH, Ban MJ, Park JH, Yoon JK, Hwang Y. Hur SS, et al. BMB Rep. 2020 Feb;53(2):74-81. doi: 10.5483/BMBRep.2020.53.2.308. BMB Rep. 2020. PMID: 31964473 Free PMC article. Review. - From mechanotransduction to extracellular matrix gene expression in fibroblasts.
Chiquet M, Gelman L, Lutz R, Maier S. Chiquet M, et al. Biochim Biophys Acta. 2009 May;1793(5):911-20. doi: 10.1016/j.bbamcr.2009.01.012. Epub 2009 Jan 31. Biochim Biophys Acta. 2009. PMID: 19339214 Review. - Force transmission at cell-cell and cell-matrix adhesions.
DeMali KA, Sun X, Bui GA. DeMali KA, et al. Biochemistry. 2014 Dec 16;53(49):7706-17. doi: 10.1021/bi501181p. Epub 2014 Dec 4. Biochemistry. 2014. PMID: 25474123 Review. - Tensile Forces and Mechanotransduction at Cell-Cell Junctions.
Charras G, Yap AS. Charras G, et al. Curr Biol. 2018 Apr 23;28(8):R445-R457. doi: 10.1016/j.cub.2018.02.003. Curr Biol. 2018. PMID: 29689229 Review. - Understanding the mechanisms of lung mechanical stress.
Garcia CS, Prota LF, Morales MM, Romero PV, Zin WA, Rocco PR. Garcia CS, et al. Braz J Med Biol Res. 2006 Jun;39(6):697-706. doi: 10.1590/s0100-879x2006000600001. Epub 2006 Jun 2. Braz J Med Biol Res. 2006. PMID: 16751974 Review.
Cited by
- Actin Cytoskeleton and Regulation of TGFβ Signaling: Exploring Their Links.
Melchionna R, Trono P, Tocci A, Nisticò P. Melchionna R, et al. Biomolecules. 2021 Feb 23;11(2):336. doi: 10.3390/biom11020336. Biomolecules. 2021. PMID: 33672325 Free PMC article. Review. - Fibronectin conformation regulates the proangiogenic capability of tumor-associated adipogenic stromal cells.
Wan AM, Chandler EM, Madhavan M, Infanger DW, Ober CK, Gourdon D, Malliaras GG, Fischbach C. Wan AM, et al. Biochim Biophys Acta. 2013 Sep;1830(9):4314-20. doi: 10.1016/j.bbagen.2013.03.033. Epub 2013 Apr 6. Biochim Biophys Acta. 2013. PMID: 23567798 Free PMC article. - Regulation of tissue fibrosis by the biomechanical environment.
Carver W, Goldsmith EC. Carver W, et al. Biomed Res Int. 2013;2013:101979. doi: 10.1155/2013/101979. Epub 2013 May 28. Biomed Res Int. 2013. PMID: 23781495 Free PMC article. Review. - How integrins control breast biology.
Glukhova MA, Streuli CH. Glukhova MA, et al. Curr Opin Cell Biol. 2013 Oct;25(5):633-41. doi: 10.1016/j.ceb.2013.06.010. Epub 2013 Jul 22. Curr Opin Cell Biol. 2013. PMID: 23886475 Free PMC article. Review. - Focal adhesion-chromatin linkage controls tumor cell resistance to radio- and chemotherapy.
Storch K, Cordes N. Storch K, et al. Chemother Res Pract. 2012;2012:319287. doi: 10.1155/2012/319287. Epub 2012 Jun 18. Chemother Res Pract. 2012. PMID: 22778951 Free PMC article.
References
- Sniadecki NJ. A tiny touch: activation of cell signaling pathways with magnetic nanoparticles. Endocrinol. 2010;151:451–457. - PubMed
- Monshausen GB, Gilroy S. Feeling green: mechanosensing in plants. Trends Cell Biol. 2009;19:228–235. - PubMed
- van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. - PubMed
Publication types
MeSH terms
Grants and funding
- U54 CA143836/CA/NCI NIH HHS/United States
- R01 CA138818‑01A1/CA/NCI NIH HHS/United States
- P50 CA058207/CA/NCI NIH HHS/United States
- U54CA143836‑01/CA/NCI NIH HHS/United States
- P50CA58207/CA/NCI NIH HHS/United States
- R01 CA138818/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources