Expression levels of estrogen receptor beta in conjunction with aromatase predict survival in non-small cell lung cancer - PubMed (original) (raw)
doi: 10.1016/j.lungcan.2011.03.009. Epub 2011 Apr 20.
Diana Marquez, Mohammad Alavi, Erin L Maresh, Li Zhang, Nam Yoon, Steve Horvath, Lora Bagryanova, Michael C Fishbein, David Chia, Richard Pietras, Lee Goodglick
Affiliations
- PMID: 21511357
- PMCID: PMC3175023
- DOI: 10.1016/j.lungcan.2011.03.009
Expression levels of estrogen receptor beta in conjunction with aromatase predict survival in non-small cell lung cancer
Vei Mah et al. Lung Cancer. 2011 Nov.
Abstract
Estrogen signaling pathways may play a significant role in the pathogenesis of non-small cell lung cancers (NSCLC) as evidenced by the expression of aromatase and estrogen receptors (ERα and ERβ) in many of these tumors. Here we examine whether ERα and ERβ levels in conjunction with aromatase define patient groups with respect to survival outcomes and possible treatment regimens. Immunohistochemistry was performed on a high-density tissue microarray with resulting data and clinical information available for 377 patients. Patients were subdivided by gender, age and tumor histology, and survival data was determined using the Cox proportional hazards model and Kaplan-Meier curves. Neither ERα nor ERβ alone was predictor of survival in NSCLC. However, when coupled with aromatase expression, higher ERβ levels predicted worse survival in patients whose tumors expressed higher levels of aromatase. Although this finding was present in patients of both genders, it was especially pronounced in women ≥ 65 years old, where higher expression of both ERβ and aromatase indicated a markedly worse survival rate than that determined by aromatase alone. Expression of ERβ together with aromatase has predictive value for survival in different gender and age subgroups of NSCLC patients. This predictive value is stronger than each individual marker alone. Our results suggest treatment with aromatase inhibitors alone or combined with estrogen receptor modulators may be of benefit in some subpopulations of these patients.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement: None declared.
Figures
Figure 1
A-C: representative staining of ERα in bronchial epithelium, adenocarcinoma and squamous carcinoma respectively; D-F: representative staining of ERβ in bronchial epithelium, adenocarcinoma and squamous carcinoma; G-I: representative staining of aromatase in bronchial epithelium, adenocarcinoma and squamous carcinoma; J: barplots of ERα and ERβ in different tumor histologies; K: barplots of ERα and ERβ in different tumor grades show a significant increase in cytoplasmic levels of ERβ with increase in grade.
Figure 1
A-C: representative staining of ERα in bronchial epithelium, adenocarcinoma and squamous carcinoma respectively; D-F: representative staining of ERβ in bronchial epithelium, adenocarcinoma and squamous carcinoma; G-I: representative staining of aromatase in bronchial epithelium, adenocarcinoma and squamous carcinoma; J: barplots of ERα and ERβ in different tumor histologies; K: barplots of ERα and ERβ in different tumor grades show a significant increase in cytoplasmic levels of ERβ with increase in grade.
Figure 1
A-C: representative staining of ERα in bronchial epithelium, adenocarcinoma and squamous carcinoma respectively; D-F: representative staining of ERβ in bronchial epithelium, adenocarcinoma and squamous carcinoma; G-I: representative staining of aromatase in bronchial epithelium, adenocarcinoma and squamous carcinoma; J: barplots of ERα and ERβ in different tumor histologies; K: barplots of ERα and ERβ in different tumor grades show a significant increase in cytoplasmic levels of ERβ with increase in grade.
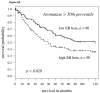
Fig 2
A: For patients with high aromatase expression (determined by staining intensity above median levels) splitting cytoplasmic ERβ expression at the median level, the Kaplan-Meier survival curve shows significantly worse survival in those patients with high ERβ (hazard ratio = 1.6, p-value = 0.029); B: Using above the 60th percentile as a cutoff to define high aromatase expression, low cytoplasmic ERβ (again median expression level) conferred a slightly better prognosis (p=0.0098, hazard ratio = 1.81).
Fig 2
A: For patients with high aromatase expression (determined by staining intensity above median levels) splitting cytoplasmic ERβ expression at the median level, the Kaplan-Meier survival curve shows significantly worse survival in those patients with high ERβ (hazard ratio = 1.6, p-value = 0.029); B: Using above the 60th percentile as a cutoff to define high aromatase expression, low cytoplasmic ERβ (again median expression level) conferred a slightly better prognosis (p=0.0098, hazard ratio = 1.81).
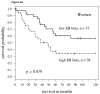
Fig 3
A: For women with high aromatase expression (staining intensity above the 60th percentile splitting cytoplasmic ERβ expression at the midpoint), the Kaplan-Meier survival curve shows worse survival in those patients with high ERβ (hazard ratio = 2.18, p-value = 0.019); B: For men, findings were similar but slightly weaker (hazard ratio = 2.04, P = 0.030). C: In women 65 and over, the findings were stronger than other population subgroups (p=0.003, hazard ratio = 3.25).
Fig 3
A: For women with high aromatase expression (staining intensity above the 60th percentile splitting cytoplasmic ERβ expression at the midpoint), the Kaplan-Meier survival curve shows worse survival in those patients with high ERβ (hazard ratio = 2.18, p-value = 0.019); B: For men, findings were similar but slightly weaker (hazard ratio = 2.04, P = 0.030). C: In women 65 and over, the findings were stronger than other population subgroups (p=0.003, hazard ratio = 3.25).
Fig 3
A: For women with high aromatase expression (staining intensity above the 60th percentile splitting cytoplasmic ERβ expression at the midpoint), the Kaplan-Meier survival curve shows worse survival in those patients with high ERβ (hazard ratio = 2.18, p-value = 0.019); B: For men, findings were similar but slightly weaker (hazard ratio = 2.04, P = 0.030). C: In women 65 and over, the findings were stronger than other population subgroups (p=0.003, hazard ratio = 3.25).
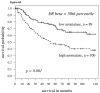
Fig 4
A: For patients with high cytoplasmic ERβ expression (determined by staining intensity above median levels) splitting aromatase expression at the median level, the Kaplan-Meier survival curve shows significantly worse survival in those patients with aromatase (hazard ratio = 1.47, p-value = 0.001); B: For women with high cytoplasmic ERβ expression, again the Kaplan-Meier survival curve again shows worse survival in those patients with higher aromatase (hazard ratio = 1.49, p-value = 0.013); C: For men, findings were similar but slightly weaker (hazard ratio = 1.38, P = 0.052).
Fig 4
A: For patients with high cytoplasmic ERβ expression (determined by staining intensity above median levels) splitting aromatase expression at the median level, the Kaplan-Meier survival curve shows significantly worse survival in those patients with aromatase (hazard ratio = 1.47, p-value = 0.001); B: For women with high cytoplasmic ERβ expression, again the Kaplan-Meier survival curve again shows worse survival in those patients with higher aromatase (hazard ratio = 1.49, p-value = 0.013); C: For men, findings were similar but slightly weaker (hazard ratio = 1.38, P = 0.052).
Fig 4
A: For patients with high cytoplasmic ERβ expression (determined by staining intensity above median levels) splitting aromatase expression at the median level, the Kaplan-Meier survival curve shows significantly worse survival in those patients with aromatase (hazard ratio = 1.47, p-value = 0.001); B: For women with high cytoplasmic ERβ expression, again the Kaplan-Meier survival curve again shows worse survival in those patients with higher aromatase (hazard ratio = 1.49, p-value = 0.013); C: For men, findings were similar but slightly weaker (hazard ratio = 1.38, P = 0.052).
Similar articles
- Prognostic relevance of estrogen receptor α, β and aromatase expression in non-small cell lung cancer.
Skjefstad K, Grindstad T, Khanehkenari MR, Richardsen E, Donnem T, Kilvaer T, Andersen S, Bremnes RM, Busund LT, Al-Saad S. Skjefstad K, et al. Steroids. 2016 Sep;113:5-13. doi: 10.1016/j.steroids.2016.05.008. Epub 2016 May 24. Steroids. 2016. PMID: 27234503 - Co-expression of estrogen receptor beta and aromatase in Japanese lung cancer patients: gender-dependent clinical outcome.
Verma MK, Miki Y, Abe K, Nagasaki S, Niikawa H, Suzuki S, Kondo T, Sasano H. Verma MK, et al. Life Sci. 2012 Oct 22;91(15-16):800-8. doi: 10.1016/j.lfs.2012.08.029. Epub 2012 Sep 6. Life Sci. 2012. PMID: 22982181 - [Prognostic value of estrogen receptors--ERalpha and ERbeta, in patients with lung cancer].
Uchikov A, Dimitrov I, Hadjiev B, Prisadov G, Uchikova E, Ganchevska P, Uzunova V. Uchikov A, et al. Khirurgiia (Sofiia). 2010;(1):32-5. Khirurgiia (Sofiia). 2010. PMID: 21972702 Bulgarian. - Estrogen receptors, antiestrogens, and non-small cell lung cancer.
Bogush TA, Dudko EA, Beme AA, Bogush EA, Kim AI, Polotsky BE, Tjuljandin SA, Davydov MI. Bogush TA, et al. Biochemistry (Mosc). 2010 Dec;75(12):1421-7. doi: 10.1134/s0006297910120011. Biochemistry (Mosc). 2010. PMID: 21314611 Review. - Estrogen receptor alpha negative breast cancer patients: estrogen receptor beta as a therapeutic target.
Skliris GP, Leygue E, Watson PH, Murphy LC. Skliris GP, et al. J Steroid Biochem Mol Biol. 2008 Mar;109(1-2):1-10. doi: 10.1016/j.jsbmb.2007.12.010. Epub 2007 Dec 8. J Steroid Biochem Mol Biol. 2008. PMID: 18243688 Review.
Cited by
- Influence of Estrogen on the NSCLC Microenvironment: A Comprehensive Picture and Clinical Implications.
Smida T, Bruno TC, Stabile LP. Smida T, et al. Front Oncol. 2020 Feb 18;10:137. doi: 10.3389/fonc.2020.00137. eCollection 2020. Front Oncol. 2020. PMID: 32133288 Free PMC article. Review. - Ribonucleotide reductase subunit M2 predicts survival in subgroups of patients with non-small cell lung carcinoma: effects of gender and smoking status.
Mah V, Alavi M, Márquez-Garbán DC, Maresh EL, Kim SR, Horvath S, Bagryanova L, Huerta-Yepez S, Chia D, Pietras R, Goodglick L. Mah V, et al. PLoS One. 2015 May 22;10(5):e0127600. doi: 10.1371/journal.pone.0127600. eCollection 2015. PLoS One. 2015. PMID: 26001082 Free PMC article. - MCT1 Modulates Cancer Cell Pyruvate Export and Growth of Tumors that Co-express MCT1 and MCT4.
Hong CS, Graham NA, Gu W, Espindola Camacho C, Mah V, Maresh EL, Alavi M, Bagryanova L, Krotee PAL, Gardner BK, Behbahan IS, Horvath S, Chia D, Mellinghoff IK, Hurvitz SA, Dubinett SM, Critchlow SE, Kurdistani SK, Goodglick L, Braas D, Graeber TG, Christofk HR. Hong CS, et al. Cell Rep. 2016 Feb 23;14(7):1590-1601. doi: 10.1016/j.celrep.2016.01.057. Epub 2016 Feb 11. Cell Rep. 2016. PMID: 26876179 Free PMC article. - Biologic roles of estrogen receptor-β and insulin-like growth factor-2 in triple-negative breast cancer.
Hamilton N, Márquez-Garbán D, Mah V, Fernando G, Elshimali Y, Garbán H, Elashoff D, Vadgama J, Goodglick L, Pietras R. Hamilton N, et al. Biomed Res Int. 2015;2015:925703. doi: 10.1155/2015/925703. Epub 2015 Mar 22. Biomed Res Int. 2015. PMID: 25874233 Free PMC article. - Expression of estrogen receptor beta and overall survival in non-small cell lung cancer patients: Protocol for a systematic review and meta-analysis of cohort studies.
Chen H, Yan M, Shi W, Shi J, Duan C, Fan Q, Wang Y, Li H. Chen H, et al. Medicine (Baltimore). 2019 Oct;98(43):e17559. doi: 10.1097/MD.0000000000017559. Medicine (Baltimore). 2019. PMID: 31651857 Free PMC article.
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
- Tanaka T, Matsuoka M, Sutani A, Gemma A, Maemondo M, et al. Frequency of and variables associated with the EGFR mutation and its subtypes. Int J Cancer. 2009 - PubMed
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. - PubMed
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. - PubMed
- Mitsudomi T, Kosaka T, Yatabe Y. Biological and clinical implications of EGFR mutations in lung cancer. Int J Clin Oncol. 2006;11:190–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA86366/CA/NCI NIH HHS/United States
- U24 CA086366/CA/NCI NIH HHS/United States
- U24 CA086366-12/CA/NCI NIH HHS/United States
- P50-CA90388/CA/NCI NIH HHS/United States
- P50 CA090388/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical