Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease - PubMed (original) (raw)
doi: 10.1016/j.jalz.2011.03.003. Epub 2011 Apr 21.
Paul S Aisen, Laurel A Beckett, David A Bennett, Suzanne Craft, Anne M Fagan, Takeshi Iwatsubo, Clifford R Jack Jr, Jeffrey Kaye, Thomas J Montine, Denise C Park, Eric M Reiman, Christopher C Rowe, Eric Siemers, Yaakov Stern, Kristine Yaffe, Maria C Carrillo, Bill Thies, Marcelle Morrison-Bogorad, Molly V Wagster, Creighton H Phelps
Affiliations
- PMID: 21514248
- PMCID: PMC3220946
- DOI: 10.1016/j.jalz.2011.03.003
Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease
Reisa A Sperling et al. Alzheimers Dement. 2011 May.
Abstract
The pathophysiological process of Alzheimer's disease (AD) is thought to begin many years before the diagnosis of AD dementia. This long "preclinical" phase of AD would provide a critical opportunity for therapeutic intervention; however, we need to further elucidate the link between the pathological cascade of AD and the emergence of clinical symptoms. The National Institute on Aging and the Alzheimer's Association convened an international workgroup to review the biomarker, epidemiological, and neuropsychological evidence, and to develop recommendations to determine the factors which best predict the risk of progression from "normal" cognition to mild cognitive impairment and AD dementia. We propose a conceptual framework and operational research criteria, based on the prevailing scientific evidence to date, to test and refine these models with longitudinal clinical research studies. These recommendations are solely intended for research purposes and do not have any clinical implications at this time. It is hoped that these recommendations will provide a common rubric to advance the study of preclinical AD, and ultimately, aid the field in moving toward earlier intervention at a stage of AD when some disease-modifying therapies may be most efficacious.
Copyright © 2011. Published by Elsevier Inc.
Figures
Fig. 1
Model of the clinical trajectory of Alzheimer's disease (AD). The stage of preclinical AD precedes mild cognitive impairment (MCI) and encompasses the spectrum of presymptomatic autosomal dominant mutation carriers, asymptomatic biomarker-positive older individuals at risk for progression to MCI due to AD and AD dementia, as well as biomarker-positive individuals who have demonstrated subtle decline from their own baseline that exceeds that expected in typical aging, but would not yet meet criteria for MCI. Note that this diagram represents a hypothetical model for the pathological-clinical continuum of AD but does not imply that all individuals with biomarker evidence of AD-pathophysiological process will progress to the clinical phases of the illness.
Fig. 2
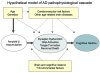
Hypothetical model of the Alzheimer's disease (AD) pathophysiological sequence leading to cognitive impairment. This model postulates that amyloid beta (Aβ) accumulation is an “upstream” event in the cascade that is associated with “downstream” synaptic dysfunction, neurodegeneration, and eventual neuronal loss. Note that although recent work from animal models suggests that specific forms of Aβ may cause both functional and morphological synaptic changes, it remains unknown whether Aβ is sufficient to incite the neurodegenerative process in sporadic late-onset AD. Age and genetics, as well as other specific host factors, such as brain and cognitive reserve, or other brain diseases may influence the response to Aβ and/or the pace of progression toward the clinical manifestations of AD.
Fig. 3
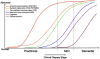
Hypothetical model of dynamic biomarkers of the AD expanded to explicate the preclinical phase: Aβ as identified by cerebrospinal fluid Aβ42 assay or PET amyloid imaging. Synaptic dysfunction evidenced by fluorodeoxyglucose (F18) positron emission tomography (FDG-PET) or functional magnetic resonance imaging (fMRI), with a dashed line to indicate that synaptic dysfunction may be detectable in carriers of the _ε_4 allele of the apolipoprotein E gene before detectable Aβ deposition. Neuronal injury is evidenced by cerebrospinal fluid tau or phospho-tau, brain structure is evidenced by structural magnetic resonance imaging. Biomarkers change from normal to maximally abnormal (y-axis) as a function of disease stage (x-axis). The temporal trajectory of two key indicators used to stage the disease clinically, cognitive and behavioral measures, and clinical function are also illustrated. Figure adapted with permission from Jack et al [22].
Fig. 4
Postulated temporal lag of approximately a decade between the deposition of Aβ (% of individuals with amyloid plaques in a large autopsy series [68]) and the clinical syndrome of AD dementia (estimated prevalence from three epidemiological studies [–71]). Figure courtesy of Mark Mintun and John Morris, Washington University.
Fig. 5
Graphic representation of the proposed staging framework for preclinical AD. Note that some individuals will not progress beyond Stage 1 or Stage 2. Individuals in Stage 3 are postulated to be more likely to progress to MCI and AD dementia. Abbreviations: AD, Alzheimer's disease; Ab, amyloid beta; PET, position emission tomography; CSF, cerebrospinal fluid; FDG, fluorodeoxyglucose, fMRI, functional magnetic resonance imaging, sMRI, structural magnetic resonance imaging.
Comment in
Similar articles
- [The new 2011 recommendations of the National Institute on Aging and the Alzheimer's Association on diagnostic guidelines for Alzheimer's disease: Preclinal stages, mild cognitive impairment, and dementia].
Croisile B, Auriacombe S, Etcharry-Bouyx F, Vercelletto M; National Institute on Aging (u.s.); Alzheimer Association. Croisile B, et al. Rev Neurol (Paris). 2012 Jun;168(6-7):471-82. doi: 10.1016/j.neurol.2011.11.007. Epub 2012 May 12. Rev Neurol (Paris). 2012. PMID: 22579080 Review. French. - Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease.
Jack CR Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH. Jack CR Jr, et al. Alzheimers Dement. 2011 May;7(3):257-62. doi: 10.1016/j.jalz.2011.03.004. Epub 2011 Apr 21. Alzheimers Dement. 2011. PMID: 21514247 Free PMC article. - The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. Albert MS, et al. Alzheimers Dement. 2011 May;7(3):270-9. doi: 10.1016/j.jalz.2011.03.008. Epub 2011 Apr 21. Alzheimers Dement. 2011. PMID: 21514249 Free PMC article. - The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. McKhann GM, et al. Alzheimers Dement. 2011 May;7(3):263-9. doi: 10.1016/j.jalz.2011.03.005. Epub 2011 Apr 21. Alzheimers Dement. 2011. PMID: 21514250 Free PMC article.
Cited by
- Screening for Early-Stage Alzheimer's Disease Using Optimized Feature Sets and Machine Learning.
Kleiman MJ, Barenholtz E, Galvin JE; Alzheimer’s Disease Neuroimaging Initiative. Kleiman MJ, et al. J Alzheimers Dis. 2021;81(1):355-366. doi: 10.3233/JAD-201377. J Alzheimers Dis. 2021. PMID: 33780367 Free PMC article. - A Western-style dietary pattern is associated with cerebrospinal fluid biomarker levels for preclinical Alzheimer's disease-A population-based cross-sectional study among 70-year-olds.
Samuelsson J, Kern S, Zetterberg H, Blennow K, Rothenberg E, Wallengren O, Skoog I, Zettergren A. Samuelsson J, et al. Alzheimers Dement (N Y). 2021 May 18;7(1):e12183. doi: 10.1002/trc2.12183. eCollection 2021. Alzheimers Dement (N Y). 2021. PMID: 34027029 Free PMC article. - Entorhinal and Transentorhinal Atrophy in Preclinical Alzheimer's Disease.
Kulason S, Xu E, Tward DJ, Bakker A, Albert M, Younes L, Miller MI. Kulason S, et al. Front Neurosci. 2020 Aug 21;14:804. doi: 10.3389/fnins.2020.00804. eCollection 2020. Front Neurosci. 2020. PMID: 32973425 Free PMC article. - Regional Hypoperfusion Predicts Decline in Everyday Functioning at Three-Year Follow-Up in Older Adults without Dementia.
Sanchez DL, Thomas KR, Edmonds EC, Bondi MW, Bangen KJ; Alzheimer’s Disease Neuroimaging Initiative. Sanchez DL, et al. J Alzheimers Dis. 2020;77(3):1291-1304. doi: 10.3233/JAD-200490. J Alzheimers Dis. 2020. PMID: 32831202 Free PMC article. - Clearance systems in the brain-implications for Alzheimer disease.
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ. Tarasoff-Conway JM, et al. Nat Rev Neurol. 2015 Aug;11(8):457-70. doi: 10.1038/nrneurol.2015.119. Epub 2015 Jul 21. Nat Rev Neurol. 2015. PMID: 26195256 Free PMC article. Review.
References
- Morris JC. Early-stage and preclinical Alzheimer disease. Alzheimer Dis Assoc Disord. 2005;19:163–5. - PubMed
- Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9:1118–27. - PubMed
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–9. - PMC - PubMed
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging– Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–9. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K24 AG035007/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- P01 AG036694/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical