NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation - PubMed (original) (raw)
NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation
Oliver Haworth et al. J Immunol. 2011.
Abstract
Immune responses are pathologically sustained in several common diseases, including asthma. To determine endogenous proresolving mechanisms for adaptive immune responses, we used a murine model of self-limited allergic airway inflammation. After cessation of allergen exposure, eosinophils and T cells were cleared concomitant with the appearance of increased numbers of NK cells in the lung and mediastinal lymph nodes. The mediastinal lymph node NK cells were activated, expressing CD27, CD11b, CD69, CD107a, and IFN-γ. NK cell depletion disrupted the endogenous resolution program, leading to delayed clearance of airway eosinophils and Ag-specific CD4(+) T cells. NK cell trafficking to inflamed tissues for resolution was dependent upon CXCR3 and CD62L. During resolution, eosinophils and Ag-specific CD4(+) T cells expressed NKG2D ligands, and a blocking Ab for the NKG2D receptor delayed clearance of these leukocytes. Of interest, NK cells expressed CMKLR1, a receptor for the proresolving mediator resolvin E1, and depletion of NK cells decreased resolvin E1-mediated resolution of allergic inflammation. Resolvin E1 regulated NK cell migration in vivo and NK cell cytotoxicity in vitro. Together, these findings indicate new functions in catabasis for NK cells that can also serve as targets for proresolving mediators in the resolution of adaptive immunity.
Figures
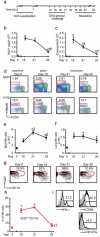
Figure 1. NK cells increase in local lymph nodes during resolution
a Mice were sensitized and aerosol challenged with OVA (as in 5) and the extent of inflammation was determined on protocol days 18 (peak inflammation), 21 and 25 (resolution). b Total number of BALF cells and c BALF eosinophils were enumerated. d Representative flow cytometry plots from MLNs and lung at baseline (day 0), peak inflammation (protocol day 18) and resolution (protocol days 21 and 25). Inserts show percentage of lymphocytes that are NK cells (NKp46+ CD3−). Time course for total NK cells in the (e) MLNs and (f) lung. g Flow cytometry plots for NK cell CD27+ CD11b+ subset during inflammation and resolution. Insets show percentages; h Percentage of CD27+ CD11b+ NK cells during the onset and resolution of allergic airway inflammation. i Representative flow cytometry plots showing the expression of IFN-γ, CD69 and CD107a on NKp46+ CD3− NK cells from the MLNs obtained in early resolution (day 21). Inset: Numbers indicate the percentage positive. Data (mean ± s.e.m) are representative of more than 3 independent experiments with n≥4 FVB mice in each group * P <0.05 (day 0) § P <0.05 (day 18), † P <0.05 (day 21).
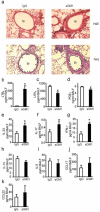
Figure 2. NK cells contribute to tissue catabasis and inflammatory mediator release
a Representative (n≥3) lung tissue sections from day 21 FVB mice given aGM1 antibody or IgG were stained with hematoxylin and eosin (H&E) or Periodic Acid Schiff (PAS). Original magnification × 200. Arrowheads denote mucus (magenta) containing Goblet cells. Br; bronchus. b-d BALF lipid mediators; LTB4, LXA4 and PGE2 e-k BALF cytokines and chemokines. Data (mean ± s.e.m) are representative of more than 3 independent experiments with n≥3 mice in each group. * P<0.05.
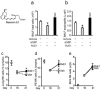
Figure 3. RvE1 mediated resolution of airway inflammation is blocked by NK cell depletion
a BALF total cells and (b) eosinophils at day 21 from mice given rabbit IgG or aGM1 plus vehicle or RvE1 (100ng; inset). The percentage of NK cells in (c) lung, (d) PB and (e) total NK cell number in MLNs from mice given vehicle or RvE1 (100ng). Data (mean ± s.e.m) are representative of more than 3 independent experiments with n≥3 mice in each group. * P<0.05.
Figure 4. Depletion of NK cells delays resolution of allergic inflammation
Mice were given aGM1 antibody or control IgG at peak inflammation (protocol day 18) and a, the total number of BALF cells (left panel) and eosinophils (right panel) were enumerated during resolution (protocol day 21). b The Ri was determined for BALF lymphocytes from FVB mice given aGM1 (red) or control IgG (white). c Representative flow cytometry plots showing percentage of CD4+ KJ1-26+ cells (inserts) from MLNs, lung, BALFs and spleen from peak inflammation (day 18) and early resolution (day 21) from mice given control IgG or aGM1. d The total number of CD4+ KJ1-26+ T cells in MLNs, lung, BALFs and spleen at peak inflammation (day 18) and following aGM1 or control IgG (day 21). e Flow cytometry plots of KJ1-26 CD4+ T cells from MLNs and lung from mice given rabbit IgG and RvE1 (100ng) or vehicle control or aGM1 and RvE1(100ng). f Total number of CD4+ KJ1-26+ T cells in MLNs and (g) lung from day 21 BALB/cj mice. Data (mean ± s.e.m) are representative of 3 experiments with n≥3 BALB/cj mice in each group. § P <0.05 (day 18) † P <0.05 (day 21).
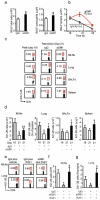
Figure 5. NK cells are recruited to MLNs and lung during resolution
a Histograms show representative expression of CXCR3 and CD62L on NK cells (NKp46+CD3−) from MLNs, lung, PB and spleen at peak inflammation (day 18, gray), and resolution (day 21 (red). b Median fluorescence intensity (MFI) of CXCR3 and CD62L expression on NK cells from the MLNs, lung, PB and spleen. c After depletion of endogenous NK cells with aGM1, CFSE labelled donor NK cells were adoptively transferred (day 19) after exposure ex vivo to anti-CXCR3, anti-CD62L, a combination of both or control IgG and enumerated in recipient mouse tissues during resolution (day 21). d MLN total cells and BALF total cell counts and eosinophils from mice reconstituted with adoptively transferred NK cells. Fold change in cxcl9 expression in (e) MLNs and (f) lung during peak inflammation (day 18) and resolution (day 21) of airway inflammation in mice given vehicle or RvE1. Data (mean ± s.e.m) are representative of more than 3 independent experiments with n≥3 FVB mice in each group. * P <0.05 (day 18); # P <0.05 (percentage compared to IgG).
Figure 6. Antigen specific T cells and eosinophils express NKG2D ligands for clearance from inflamed lung
a Expression of NKG2D ligands on KJ1-26+ and KJ1-26− CD4+ T cells from MLNs, BALFs, spleen and lung eosinophils (Eos) was determined with NKG2D-Fc fusion protein. Histograms show secondary alone (gray) and expression of ligands (black). Inserts show the delta (Δ) median fluorescence intensity (MFI-MFI control) and the percentage of cells positive for NKG2D ligands. Mice were depleted of endogenous NK cells with aGM1 and reconstituted with donor NK cells that were exposed ex vivo to anti-NKG2D (aNKG2D). b BALF total cells and eosinophils were enumerated after aNKG2D or IgG control antibody. c Flow cytometry plots from CD4+ T cells in MLNs, BALFs, spleen and lung. Inserts show percentages of CD4+ KJ1-26+ T cells. d The number of CD4+ KJ1-26+ T cells in MLNs, BALFs, spleen and lung (percentage) after aNKG2D or control antibody. e Representative histograms show expression of the RvE1 receptor CMKLR1 on NK cells (NKp46+ CD3−) from MLNs and lung (day 21). f NK cell cytotoxicity towards RMA/S target cells was determined in the presence of RvE1 (10 nM) or vehicle control * P <0.05 (vehicle), § P <0.05 (RvE1). Data (mean ± s.e.m) are representative of more than 3 independent experiments with n≥4 BALB/cj mice in each group.
Similar articles
- Allergen-specific CTL require perforin expression to suppress allergic airway inflammation.
Enomoto N, Hyde E, Ma JZ, Yang J, Forbes-Blom E, Delahunt B, Le Gros G, Ronchese F. Enomoto N, et al. J Immunol. 2012 Feb 15;188(4):1734-41. doi: 10.4049/jimmunol.1102699. Epub 2012 Jan 16. J Immunol. 2012. PMID: 22250087 - Natural killer cells accumulate in lung-draining lymph nodes and regulate airway eosinophilia in a murine model of asthma.
Ple C, Barrier M, Amniai L, Marquillies P, Bertout J, Tsicopoulos A, Walzer T, Lassalle P, Duez C. Ple C, et al. Scand J Immunol. 2010 Aug;72(2):118-27. doi: 10.1111/j.1365-3083.2010.02419.x. Scand J Immunol. 2010. PMID: 20618770 - Pro-inflammatory role of natural killer cells in the development of allergic airway disease.
Mathias CB, Guernsey LA, Zammit D, Brammer C, Wu CA, Thrall RS, Aguila HL. Mathias CB, et al. Clin Exp Allergy. 2014 Apr;44(4):589-601. doi: 10.1111/cea.12271. Clin Exp Allergy. 2014. PMID: 24397722 Free PMC article. - Re-defining the unique roles for eosinophils in allergic respiratory inflammation.
Jacobsen EA, Lee NA, Lee JJ. Jacobsen EA, et al. Clin Exp Allergy. 2014 Sep;44(9):1119-36. doi: 10.1111/cea.12358. Clin Exp Allergy. 2014. PMID: 24961290 Free PMC article. Review. - Subsets of human natural killer cells and their regulatory effects.
Fu B, Tian Z, Wei H. Fu B, et al. Immunology. 2014 Apr;141(4):483-9. doi: 10.1111/imm.12224. Immunology. 2014. PMID: 24303897 Free PMC article. Review.
Cited by
- Innate immunity is a key factor for the resolution of inflammation in asthma.
Barnig C, Levy BD. Barnig C, et al. Eur Respir Rev. 2015 Mar;24(135):141-53. doi: 10.1183/09059180.00012514. Eur Respir Rev. 2015. PMID: 25726564 Free PMC article. Review. - NK cells modulate the inflammatory response to corneal epithelial abrasion and thereby support wound healing.
Liu Q, Smith CW, Zhang W, Burns AR, Li Z. Liu Q, et al. Am J Pathol. 2012 Aug;181(2):452-62. doi: 10.1016/j.ajpath.2012.04.010. Epub 2012 Jun 22. Am J Pathol. 2012. PMID: 22728064 Free PMC article. - Maternal diesel particle exposure promotes offspring asthma through NK cell-derived granzyme B.
Qian Q, Chowdhury BP, Sun Z, Lenberg J, Alam R, Vivier E, Gorska MM. Qian Q, et al. J Clin Invest. 2020 Aug 3;130(8):4133-4151. doi: 10.1172/JCI130324. J Clin Invest. 2020. PMID: 32407293 Free PMC article. - Natural Killer Cells and Host Defense Against Human Rhinoviruses Is Partially Dependent on Type I IFN Signaling.
van der Heide SL, Xi Y, Upham JW. van der Heide SL, et al. Front Cell Infect Microbiol. 2020 Oct 21;10:510619. doi: 10.3389/fcimb.2020.510619. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33194777 Free PMC article. - Resolution phase lipid mediators of inflammation: agonists of resolution.
Serhan CN, Chiang N. Serhan CN, et al. Curr Opin Pharmacol. 2013 Aug;13(4):632-40. doi: 10.1016/j.coph.2013.05.012. Epub 2013 Jun 6. Curr Opin Pharmacol. 2013. PMID: 23747022 Free PMC article. Review.
References
- Nathan C, Ding A. Nonresolving inflammation. Cell. 140:871–882. - PubMed
- Busse WW, Lemanske RF., Jr. Asthma. N Engl J Med. 2001;344:350–362. - PubMed
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous