Hypothesis on the dual origin of the Mammalian subplate - PubMed (original) (raw)
Hypothesis on the dual origin of the Mammalian subplate
Juan F Montiel et al. Front Neuroanat. 2011.
Abstract
The development of the mammalian neocortex relies heavily on subplate. The proportion of this cell population varies considerably in different mammalian species. Subplate is almost undetectable in marsupials, forms a thin, but distinct layer in mouse and rat, a larger layer in carnivores and big-brained mammals as pig, and a highly developed embryonic structure in human and non-human primates. The evolutionary origin of subplate neurons is the subject of current debate. Some hypothesize that subplate represents the ancestral cortex of sauropsids, while others consider it to be an increasingly complex phylogenetic novelty of the mammalian neocortex. Here we review recent work on expression of several genes that were originally identified in rodent as highly and differentially expressed in subplate. We relate these observations to cellular morphology, birthdating, and hodology in the dorsal cortex/dorsal pallium of several amniote species. Based on this reviewed evidence we argue for a third hypothesis according to which subplate contains both ancestral and newly derived cell populations. We propose that the mammalian subplate originally derived from a phylogenetically ancient structure in the dorsal pallium of stem amniotes, but subsequently expanded with additional cell populations in the synapsid lineage to support an increasingly complex cortical plate development. Further understanding of the detailed molecular taxonomy, somatodendritic morphology, and connectivity of subplate in a comparative context should contribute to the identification of the ancestral and newly evolved populations of subplate neurons.
Keywords: Monodelphis domestica; cerebral cortex evolution; chick; human; pig; subplate; turtle.
Figures
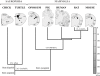
Figure 1
Cladogram of the species investigated with subplate murine markers defined by fossil record, nuclear and mitochondrial DNA sequence data. This is based on previous literature (Carroll, ; Mannen and Li, ; Rieppel and Reisz, ; Zardoya and Meyer, ; Gibbs et al., ; Pereira and Baker, 2006). Amniotes group is early subdivided into sauropsids (reptiles and birds) and mammals, both lines are represented in analyzed species. The brains of mammals and sauropsids, sketched in coronal sections, are morphologically very different, although being organized according to a common basic plan, which is more evident at developmental stages. Ag, amygdala; DC, dorsal cortex; DP, dorsal pallium; DVR, dorsal ventricular ridge; H, hippocampus; HP, hyperpallium; LC, lateral cortex; LP, lateral pallium; M, mesopallium; MC, medial cortex; N, nidopallium; OC, olfactory cortex; S, striatum; SEP, septum; VP, ventral pallium.
Figure 2
Comparative expression of murine subplate markers (Ctgf, Moxd1, and Nurr1) in selected species. (A–C) mRNA expression of Ctgf and Moxd1 and protein expression of Nurr1 in the adult turtle, with external plexiform layer (EPL), cell dense layer (CDL), and internal plexiform layer (IPL) indicated. All three murine subplate markers are expressed in the dense cell layer in turtle. (D–F) mRNA expression of Ctgf and Moxd1 and protein expression of Nurr1 in chick dorsal pallium with the hyperpallium (H) and Mesopallium (M) indicated. Ctgf is expressed in a column within hyperpallium while Moxd1 labels scattered cells in the hyperpallium, across columnar boundaries. Similarly, Nurr1 is expressed in the dorsal most tip of the hyperpallium, across several columns, but not along their entire depth. (G–I) mRNA expression of Ctgf and Moxd1 and protein expression of Nurr1 in postnatal opossum cortex with cortical plate and marginal zone indicated. Ctgf and Moxd1 are expressed at in the upper cortical plate at the junction with the marginal zone at P20 while Nurr1 is primarily expressed in the lower cortical plate at P44. (J,K) Protein expression of Ctgf and Nurr1 in the embryonic pig cortex with subplate, cortical plate, and marginal zone indicated. Ctgf protein is localized to a thin band within the subplate, while Nurr1 protein is localized to a thicker band representing the subplate and possibly the lower parts of cortical plate. Nurr1+ cells follow the up and down of the above lying cortical gyri and sulci (at the edges of the image). (L,N,P) mRNA expression of Ctgf and Moxd1 and protein expression of Nurr1 in the postnatal mouse cortex with subplate, layers II–VI, and marginal zone indicated. All three markers are confined to the subplate zone in mice. (M,O,Q) mRNA expression of Ctgf and Moxd1 and protein expression of Nurr1 in postnatal rat cortex with subplate, layers II–VI, and marginal zone indicated. Ctgf and Nurr1 expression is confined to the subplate zone while Moxd1 expression is absent in the rat cortex [see inset in (N) (mouse) and (O) (rat)]. Scale bars = 200 μm.
Figure 3
Schematic diagram summarizes the distribution and overlap of several murine subplate cell markers and other neuronal markers in rodents and their distribution in turtle. (A) In early postnatal mouse cerebral cortex Cplx3 and Ctgf are exclusively expressed in subplate cells. Nurr1 has additional expression in layer VI. These markers partially colocalize. Foxp2 is only expressed in layer VI and the overlap with Nurr1 is not yet investigated. Ddc immunoreactive cells are scattered into layer VI and white matter in addition to subplate and do not overlap with either Nurr1 or Cplx3. Calretinin and GABA immunoreactive cells appear in the whole cortical thickness. Neither Cplx3, Ctgf and Nurr1, nor Ddc expressing subplate cells express GABA. (B) In turtle dorsal cortex, the murine SP markers (Cplx3, Ctgf, and Nurr1) and Moxd1 are present at the upper part of the cell dense layer. Unfortunately there is no data about potential colocalization between markers in turtle cortex at present. FoxP2 is expressed below the subplate markers, in the lower part of the cell dense layer. Further, ubiquitous mammalian cortical markers appear restricted to either the internal plexiform layer (GAD65/67 and GABA) or the external plexiform layer (calretinin).
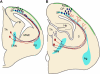
Figure 4
Functional correlation between the developing thalamo-cortical projections, cortical SP zone, and thalamic reticular nucleus. (A) Corticofugal (blue) and thalamo-cortical (red) axons extend toward each other at early stages during embryonic development and reach close to their targets. However they both stop short of their ultimate targets and corticofugal projections from subplate and layer VI accumulate in the thalamic reticular nucleus (TRN) and thalamo-cortical projections in subplate, respectively. Both compartments contain largely transient cells that get integrated into circuits during these early stages. (B) Toward the middle of the first postnatal week corticofugal and corticopetal axons enter the thalamus (Th) and cortical plate (CP), respectively, where they arborize and establish their contacts with their ultimate targets in thalamus and neocortex. There are signs of fiber decussations in the TRN and in the subplate indicating some rearrangements during development. Pale blue areas (amygdala, subplate, and thalamic reticular nucleus) represent brain regions sharing gene expression patterns. Ag, amygdala; Hp, hippocampus; MGE, medial ganglionic eminence; MZ, marginal zone (green area); S, striatum.
Similar articles
- The evolution of the dorsal pallium in the telencephalon of amniotes: cladistic analysis and a new hypothesis.
Butler AB. Butler AB. Brain Res Brain Res Rev. 1994 Jan;19(1):66-101. doi: 10.1016/0165-0173(94)90004-3. Brain Res Brain Res Rev. 1994. PMID: 8167660 Review. - Comparative aspects of subplate zone studied with gene expression in sauropsids and mammals.
Wang WZ, Oeschger FM, Montiel JF, García-Moreno F, Hoerder-Suabedissen A, Krubitzer L, Ek CJ, Saunders NR, Reim K, Villalón A, Molnár Z. Wang WZ, et al. Cereb Cortex. 2011 Oct;21(10):2187-203. doi: 10.1093/cercor/bhq278. Epub 2011 Mar 2. Cereb Cortex. 2011. PMID: 21368089 - In search of common developmental and evolutionary origin of the claustrum and subplate.
Bruguier H, Suarez R, Manger P, Hoerder-Suabedissen A, Shelton AM, Oliver DK, Packer AM, Ferran JL, García-Moreno F, Puelles L, Molnár Z. Bruguier H, et al. J Comp Neurol. 2020 Dec 1;528(17):2956-2977. doi: 10.1002/cne.24922. Epub 2020 May 6. J Comp Neurol. 2020. PMID: 32266722 Review. - Ancestry of the mammalian preplate and its derivatives: evolutionary relicts or embryonic adaptations?
Aboitiz F, Montiel J, García RR. Aboitiz F, et al. Rev Neurosci. 2005;16(4):359-76. doi: 10.1515/revneuro.2005.16.4.359. Rev Neurosci. 2005. PMID: 16519011 Review. - Origin and fate of fetuin-containing neurons in the developing neocortex of the fetal sheep.
Saunders NR, Habgood MD, Ward RA, Reynolds ML. Saunders NR, et al. Anat Embryol (Berl). 1992 Oct;186(5):477-86. doi: 10.1007/BF00185461. Anat Embryol (Berl). 1992. PMID: 1280010
Cited by
- Development of reciprocal connections between the dorsal lateral geniculate nucleus and the thalamic reticular nucleus.
Campbell PW, Govindaiah G, Guido W. Campbell PW, et al. Neural Dev. 2024 Jun 18;19(1):6. doi: 10.1186/s13064-024-00183-5. Neural Dev. 2024. PMID: 38890758 Free PMC article. - Temporal origin of mouse claustrum and development of its cortical projections.
Hoerder-Suabedissen A, Ocana-Santero G, Draper TH, Scott SA, Kimani JG, Shelton AM, Butt SJB, Molnár Z, Packer AM. Hoerder-Suabedissen A, et al. Cereb Cortex. 2023 Mar 21;33(7):3944-3959. doi: 10.1093/cercor/bhac318. Cereb Cortex. 2023. PMID: 36104852 Free PMC article. - Subplate Neurons as an Organizer of Mammalian Neocortical Development.
Ohtaka-Maruyama C. Ohtaka-Maruyama C. Front Neuroanat. 2020 Mar 19;14:8. doi: 10.3389/fnana.2020.00008. eCollection 2020. Front Neuroanat. 2020. PMID: 32265668 Free PMC article. - Genetic Elimination of Connective Tissue Growth Factor in the Forebrain Affects Subplate Neurons in the Cortex and Oligodendrocytes in the Underlying White Matter.
Yu IS, Chang HC, Chen KC, Lu YL, Shy HT, Chen CY, Lee KY, Lee LJ. Yu IS, et al. Front Neuroanat. 2019 Feb 20;13:16. doi: 10.3389/fnana.2019.00016. eCollection 2019. Front Neuroanat. 2019. PMID: 30842729 Free PMC article.
References
- Abellán A., Legaz I., Vernier B., Retaux S., Medina L. (2009). Olfactory and amygdalar structures of the chicken ventral pallium based on the combinatorial expression patterns of LIM and other developmental regulatory genes. J. Comp. Neurol. 516, 166–186 - PubMed
- Aboitiz F., Montiel J. (2007). Origin and evolution of the vertebrate telencephalon, with special reference to the mammalian neocortex. Adv. Anat. Embryol. Cell. Biol. 193, 1–112 - PubMed
- Aboitiz F., Montiel J., Garcia R. R. (2005). Ancestry of the mammalian preplate and its derivatives: evolutionary relicts or embryonic adaptations? Rev. Neurosci. 16, 359–376 - PubMed
- Allendoerfer K. L., Shatz C. J. (1994). The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu. Rev. Neurosci. 17, 185–218 - PubMed
LinkOut - more resources
Full Text Sources