Doc2 supports spontaneous synaptic transmission by a Ca(2+)-independent mechanism - PubMed (original) (raw)
Doc2 supports spontaneous synaptic transmission by a Ca(2+)-independent mechanism
Zhiping P Pang et al. Neuron. 2011.
Abstract
Two families of Ca(2+)-binding proteins have been proposed as Ca(2+) sensors for spontaneous release: synaptotagmins and Doc2s, with the intriguing possibility that Doc2s may represent high-affinity Ca(2+) sensors that are activated by deletion of synaptotagmins, thereby accounting for the increased spontaneous release in synaptotagmin-deficient synapses. Here, we use an shRNA-dependent quadruple knockdown of all four Ca(2+)-binding proteins of the Doc2 family to confirm that Doc2-deficient synapses exhibit a marked decrease in the frequency of spontaneous release events. Knockdown of Doc2s in synaptotagmin-1-deficient synapses, however, failed to reduce either the increased spontaneous release or the decreased evoked release of these synapses, suggesting that Doc2s do not constitute Ca(2+) sensors for asynchronous release. Moreover, rescue experiments revealed that the decrease in spontaneous release induced by the Doc2 knockdown in wild-type synapses is fully reversed by mutant Doc2B lacking Ca(2+)-binding sites. Thus, our data suggest that Doc2s are modulators of spontaneous synaptic transmission that act by a Ca(2+)-independent mechanism.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
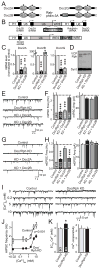
Figure 1. Knockdown of Doc2 proteins reduces spontaneous ‘mini’ release
A. Domain structures of Doc2 proteins and rabphilin-3A. Note that rabphilin resembles a Doc2 protein with the characteristic C2-domains but an extra N-terminal Zinc-finger domain (black dots = predicted Ca2+-binding sites). B. Lentiviral system for KD of all four members of the Doc2/rabphilin protein family (H1 and U6 = human H1 and U6 pol III promoters; Ub = ubiquitin pol II promoter; WRE = woodchuck hepatitis virus regulatory element). C & D. Measurement of KD efficiency. Cortical neurons cultured from newborn mice were co-infected at 4 days in vitro (DIV4) with a control lentivirus expressing only EGFP (Control), with the two lentiviruses described in panel B either without a rescue cDNA (Doc/Rph KD), or with an shRNA-resistant rescue cDNA encoding Doc2B (KD + Doc2B). Cells were harvested at DIV14, and mRNA levels for the three Doc2 isoforms were measured by quantitative rt-PCR (C), whereas the protein levels for rabphilin were assessed by immunoblotting (D; Synt. = syntaxin; VCP = vasolin-containing protein (loading controls)). E & F. Representative traces (E) and summary graphs of the frequency (F, left) and amplitude (F, right) of inhibitory mIPSCs monitored in control neurons (Control) and Doc2/ rabphilin KD neurons without (Doc/Rph KD) or with expression of Doc2A or Doc2B rescue cDNA (KD + Doc2A or + Doc2B). G & H. Same as E & F, except that excitatory mEPSCs were recorded. I. Representative traces of mIPSCs monitored at different external Ca2+-concentrations in cortical neurons infected with control and Doc2/rabphilin KD lentiviruses. J. Plot of the mIPSC frequency as a function of the external Ca2+-concentration. K. Apparent Ca2+-affinity (left, estimated as the EC50 for the mIPSC frequency) and Ca2+-cooperativity (right) of spontaneous mIPSCs in control and Doc2/rabphilin KD neurons, calculated by Hill function fits of individual Ca2+-titration experiments. Data shown are means ± SEMs (in C, n=3 culture experiments; in F, H, and K, number of cells/experiments analyzed are shown in bars; n for J = K; statistical analyses for F, H, and K are by Student’s t-test [*=p<0.05 and ***=p<0.001], and for J by two-way ANOVA). See also Figure S1.
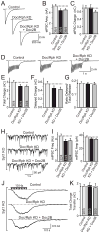
Figure 2. Doc2/rabphilin KD does not change evoked synchronous or asynchronous release
A–C. Representative traces (A) and summary graphs of the amplitude (B) and charge transfer (C) of IPSCs evoked by isolated action potentials in control neurons and Doc2/rabphilin KD neurons without or with Doc2B rescue. Neuronal KDs were performed as described for Figure 1C. D–G. Representative traces (D), total charge transfer (E), charge transfer during delayed release (F), and the ratio of delayed to total release measured by charge transfer (G) of IPSCs evoked by a 10 Hz stimulus train applied for 1 s. H & I. Representative traces (H) and the frequency (I, left) or amplitude (I, right) of mIPSCs monitored in cortical neurons cultured from Syt1 KO mice and infected with control lentivirus or the Doc2/rabphilin KD lentiviruses without or with Doc2B rescue as described in Figure 1C. For mEPSCs, see Fig. S2. J & K. Representative traces (J) and total charge transfer (K) of IPSCs evoked by a 1 sec 10 Hz stimulus train in Syt1 KO neurons infected with control or KD lentiviruses as above. Data shown are means ± SEMs (number of cells/experiments analyzed are shown in the bars; Student’s t-test failed to detect significant differences). See also Figure S2.
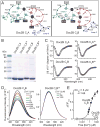
Figure 3. Ca2+-binding deficient mutant C2-domains of Doc2B are folded
A. Schema of the predicted Ca2+-binding sites of the Doc2B C2A- (left) and C2B-domain Ca2+-binding sites (right), based on the atomic structures of the rabphilin C2B-domain and the Syt1 C2A- and C2B-domains (Fernandez-Chacon et al., 2001; Fernandez et al., 2001; Ubach et al., 1999). Aspartate and glutamate residues involved Ca2+-binding are boxed; residues substituted for alanines in the Ca2+-binding site mutants are shown on a black background. B. Purified wild-type or mutant Doc2B C2-domains. The mutant Doc2B C2A- and C2B-domains contain three alanine substitutions each in critical Ca2+-binding residues (C2A3A: D163A, D218A and D220A; C2B3A: D303A, D357A and D359A). C. Circular dichroism spectra of wild type and mutant Doc2B C2-domains. D & E. Ca2+-titration of intrinsic tryptophan fluorescence of wild-type and mutant Doc2B C2B-domains. Panel D depicts fluorescence spectra of the wild-type (left) and mutant Doc2 C2B-domain (right) as a function of increasing concentrations of free Ca2+, followed by addition of excess EGTA (5 mM) to remove bound Ca2+. Panel F plots the fluorescence changes (ΔF/Fmax) as a function of the free Ca2+-concentration on a semi-logarithmic scale (note that 0 Ca2+ is plotted here at ~10−5 μM for illustration purposes). Data are representative experiments (C-E) or means ± SEMs (F, n=3 independent experiments). See also Figure S3.
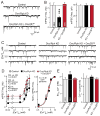
Figure 4. Ca2+-binding deficient Doc2B rescues the decrease in miniature IPSC frequency in Doc2 KD neurons
A & B. Representative traces (A) and summary graphs of the frequency (B, left) and amplitude (B, right) of mIPSCs monitored in control neurons (Control) and Doc2/rabphilin KD neurons without (Doc/Rph KD) or with expression of mutant Doc2B (KD + Doc2B6A) in which all Ca2+-binding sites were ablated (see Figure 3A). C. Representative traces of mIPSCs monitored at different external Ca2+-concentrations in cortical neurons infected with control lentivirus and Doc2/rabphilin KD lentiviruses without or with expression of mutant Doc2B6A rescue cDNA. D. Plot of the mean absolute (left) and normalized mIPSC frequency (right) as a function of the external Ca2+-concentration. mIPSCs were monitored in control infected neurons and Doc2/rabphilin KD neurons without and with rescue with mutant Doc2B6A as described in C. E. Apparent Ca2+-affinity (left, estimated as the EC50 for the mIPSC frequency) and Ca2+-cooperativity (right) of spontaneous mIPSCs in control and Doc2/rabphilin KD neurons without or with rescue by mutant Doc2B6A, as calculated from Hill function fits of individual Ca2+-titration experiments. Data shown are means ± SEMs (number of cells/experiments analyzed are shown in the bars; in D, n corresponds to E; statistical analyses for B and E are by Student’s t-test [*=p<0.05 and ***=p<0.001], and for D by two-way ANOVA).
Similar articles
- Ca2+ sensor proteins in spontaneous release and synaptic plasticity: Limited contribution of Doc2c, rabphilin-3a and synaptotagmin 7 in hippocampal glutamatergic neurons.
Bourgeois-Jaarsma Q, Miaja Hernandez P, Groffen AJ. Bourgeois-Jaarsma Q, et al. Mol Cell Neurosci. 2021 Apr;112:103613. doi: 10.1016/j.mcn.2021.103613. Epub 2021 Mar 19. Mol Cell Neurosci. 2021. PMID: 33753311 - Doc2 Proteins Are Not Required for the Increased Spontaneous Release Rate in Synaptotagmin-1-Deficient Neurons.
Díez-Arazola R, Meijer M, Bourgeois-Jaarsma Q, Cornelisse LN, Verhage M, Groffen AJ. Díez-Arazola R, et al. J Neurosci. 2020 Mar 25;40(13):2606-2617. doi: 10.1523/JNEUROSCI.0309-19.2020. Epub 2020 Feb 25. J Neurosci. 2020. PMID: 32098902 Free PMC article. - Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release.
Groffen AJ, Martens S, Díez Arazola R, Cornelisse LN, Lozovaya N, de Jong AP, Goriounova NA, Habets RL, Takai Y, Borst JG, Brose N, McMahon HT, Verhage M. Groffen AJ, et al. Science. 2010 Mar 26;327(5973):1614-8. doi: 10.1126/science.1183765. Epub 2010 Feb 11. Science. 2010. PMID: 20150444 Free PMC article. - [Doc2 as a presynaptic modulator of Ca(2+)-dependent neurotransmitter release].
Orita S, Sasaki T, Takai Y. Orita S, et al. Seikagaku. 1999 Jul;71(7):530-5. Seikagaku. 1999. PMID: 10483292 Review. Japanese. No abstract available. - Roles of SNARE proteins and synaptotagmin I in synaptic transmission: studies at the Drosophila neuromuscular synapse.
Kidokoro Y. Kidokoro Y. Neurosignals. 2003 Jan-Feb;12(1):13-30. doi: 10.1159/000068912. Neurosignals. 2003. PMID: 12624525 Review.
Cited by
- Ferlins and TgDOC2 in Toxoplasma Microneme, Rhoptry and Dense Granule Secretion.
Tagoe DNA, Drozda AA, Falco JA, Bechtel TJ, Weerapana E, Gubbels MJ. Tagoe DNA, et al. Life (Basel). 2021 Mar 9;11(3):217. doi: 10.3390/life11030217. Life (Basel). 2021. PMID: 33803212 Free PMC article. - Doc2b synchronizes secretion from chromaffin cells by stimulating fast and inhibiting sustained release.
Pinheiro PS, de Wit H, Walter AM, Groffen AJ, Verhage M, Sørensen JB. Pinheiro PS, et al. J Neurosci. 2013 Oct 16;33(42):16459-70. doi: 10.1523/JNEUROSCI.2656-13.2013. J Neurosci. 2013. PMID: 24133251 Free PMC article. - Mutations that disrupt Ca²⁺-binding activity endow Doc2β with novel functional properties during synaptic transmission.
Gaffaney JD, Xue R, Chapman ER. Gaffaney JD, et al. Mol Biol Cell. 2014 Feb;25(4):481-94. doi: 10.1091/mbc.E13-10-0571. Epub 2013 Dec 19. Mol Biol Cell. 2014. PMID: 24356452 Free PMC article. - Loss of Doc2-Dependent Spontaneous Neurotransmission Augments Glutamatergic Synaptic Strength.
Ramirez DMO, Crawford DC, Chanaday NL, Trauterman B, Monteggia LM, Kavalali ET. Ramirez DMO, et al. J Neurosci. 2017 Jun 28;37(26):6224-6230. doi: 10.1523/JNEUROSCI.0418-17.2017. Epub 2017 May 24. J Neurosci. 2017. PMID: 28539418 Free PMC article. - Calcium dependence of spontaneous neurotransmitter release.
Williams CL, Smith SM. Williams CL, et al. J Neurosci Res. 2018 Mar;96(3):335-347. doi: 10.1002/jnr.24116. Epub 2017 Jul 12. J Neurosci Res. 2018. PMID: 28699241 Free PMC article. Review.
References
- Brose N, Petrenko AG, Südhof TC, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. - PubMed
- Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Südhof TC, Rizo J. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. - PubMed
- Davletov BA, Südhof TC. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J Biol Chem. 1993;268:26386–26390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH089054/MH/NIMH NIH HHS/United States
- F32 NS067896/NS/NINDS NIH HHS/United States
- 1F32NS067896/NS/NINDS NIH HHS/United States
- 1R01 MH089054/MH/NIMH NIH HHS/United States
- P50 MH086403/MH/NIMH NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous