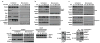TLR signalling augments macrophage bactericidal activity through mitochondrial ROS - PubMed (original) (raw)
TLR signalling augments macrophage bactericidal activity through mitochondrial ROS
A Phillip West et al. Nature. 2011.
Abstract
Reactive oxygen species (ROS) are essential components of the innate immune response against intracellular bacteria and it is thought that professional phagocytes generate ROS primarily via the phagosomal NADPH oxidase machinery. However, recent studies have suggested that mitochondrial ROS (mROS) also contribute to mouse macrophage bactericidal activity, although the mechanisms linking innate immune signalling to mitochondria for mROS generation remain unclear. Here we demonstrate that engagement of a subset of Toll-like receptors (TLR1, TLR2 and TLR4) results in the recruitment of mitochondria to macrophage phagosomes and augments mROS production. This response involves translocation of a TLR signalling adaptor, tumour necrosis factor receptor-associated factor 6 (TRAF6), to mitochondria, where it engages the protein ECSIT (evolutionarily conserved signalling intermediate in Toll pathways), which is implicated in mitochondrial respiratory chain assembly. Interaction with TRAF6 leads to ECSIT ubiquitination and enrichment at the mitochondrial periphery, resulting in increased mitochondrial and cellular ROS generation. ECSIT- and TRAF6-depleted macrophages have decreased levels of TLR-induced ROS and are significantly impaired in their ability to kill intracellular bacteria. Additionally, reducing macrophage mROS levels by expressing catalase in mitochondria results in defective bacterial killing, confirming the role of mROS in bactericidal activity. These results reveal a novel pathway linking innate immune signalling to mitochondria, implicate mROS as an important component of antibacterial responses and further establish mitochondria as hubs for innate immune signalling.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
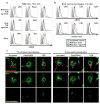
Figure 1. TLR1/2/4 signaling induces mROS generation and mitochondrial recruitment to phagosomes
a, RAW cells stimulated as indicated, stained with MitoSOX [mROS], and analyzed by FACS. b, BMM were stimulated as indicated, stained with MitoSOX (top panels) or CM-H2DCFDA [cellular H2O2] (bottom panels), and analyzed by FACS. c, BMM were incubated with uncoated, Pam3CSK4-, or LPS-coated latex beads and mitochondrial networks were immunostained with HSP70 antibodies [Mito]. Confocal Z-stacks were acquired and colocalized beads (red pixels) and mitochondria (green pixels) are displayed in yellow (bottom). Images shown are representative of approximately 100 cells analyzed.
Figure 2. TRAF6 is recruited to mitochondria upon TLR1/2/4, but not TLR3/9, signaling to engage ECSIT on the mitochondrial surface
a-e, RAW cells were stimulated with TLR agonists for the indicated times, cells were fractionated, and extracts were blotted. Purified mitochondrial lysates were immunoprecipitated with ECSIT antibody overnight (d). Heavy chain IgG is indicated by an asterisk (d). Equal amounts of extracts were treated with the indicated amount of proteinase K on ice with or without 0.2% saponin to gently permeabilize mitochondrial membranes (e).
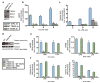
Figure 3. TRAF6-ECSIT signaling regulates generation of mitochondrial and cellular ROS, which requires TRAF6 E3-ubiquitin ligase activity
a-c, WT or ECSIT +/- BMM were transduced with shRNAs and left untreated or stimulated with LPS. Untreated BMM were lysed and extracts blotted for TRAF6 and ECSIT (a). Cells were stained with MitoSOX (b) or CM-H2DCFDA (c) and analyzed by FACS. d-f, WT or TRAF6 null BMM were left untreated or transduced with TRAF6 expressing retroviruses, then stimulated with for the indicated times. Unstimulated BMM were lysed and extracts blotted for TRAF6 expression (d). Cells were stained with MitoSOX (e) and CM-H2DCFDA (f) and analyzed by FACS. Error bars represent s.d. of the mean from triplicate samples.
Figure 4. ECSIT depleted and MCAT transgenic macrophages are less effective at clearing Salmonella
a-c, BMM from WT or ECSIT +/- were transduced with shRNAs and infected with GFP-Salmonella. Cells were fixed and stained with Dapi (a), solubilized in SDS (b), or lysed and plated (c). d-e, WT or MCAT BMM were infected with GFP-Salmonella. Cells were solubilized in SDS (d) or fixed and Dapi stained (e). Triplicate wells were pooled and blotted (b, d), and error bars (c) represent s.d. from triplicate samples. f-g, WT (n=6), MCAT (n=5), and ECSIT +/- (n=5) mice were infected with Salmonella i.p. Five days post infection, spleens (f) and livers (g) were homogenized and CFUs per gram of tissue were determined. Error bars indicate s.e.m., and p values are relative to WT.
Similar articles
- Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity.
Geng J, Sun X, Wang P, Zhang S, Wang X, Wu H, Hong L, Xie C, Li X, Zhao H, Liu Q, Jiang M, Chen Q, Zhang J, Li Y, Song S, Wang HR, Zhou R, Johnson RL, Chien KY, Lin SC, Han J, Avruch J, Chen L, Zhou D. Geng J, et al. Nat Immunol. 2015 Nov;16(11):1142-52. doi: 10.1038/ni.3268. Epub 2015 Sep 28. Nat Immunol. 2015. PMID: 26414765 Free PMC article. - Peroxiredoxin-6 Negatively Regulates Bactericidal Activity and NF-κB Activity by Interrupting TRAF6-ECSIT Complex.
Min Y, Wi SM, Shin D, Chun E, Lee KY. Min Y, et al. Front Cell Infect Microbiol. 2017 Mar 24;7:94. doi: 10.3389/fcimb.2017.00094. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28393051 Free PMC article. - An Essential Role for ECSIT in Mitochondrial Complex I Assembly and Mitophagy in Macrophages.
Carneiro FRG, Lepelley A, Seeley JJ, Hayden MS, Ghosh S. Carneiro FRG, et al. Cell Rep. 2018 Mar 6;22(10):2654-2666. doi: 10.1016/j.celrep.2018.02.051. Cell Rep. 2018. PMID: 29514094 Free PMC article. - Mitochondria in innate immune signaling.
Banoth B, Cassel SL. Banoth B, et al. Transl Res. 2018 Dec;202:52-68. doi: 10.1016/j.trsl.2018.07.014. Epub 2018 Aug 7. Transl Res. 2018. PMID: 30165038 Free PMC article. Review. - Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance.
Nguyen GT, Green ER, Mecsas J. Nguyen GT, et al. Front Cell Infect Microbiol. 2017 Aug 25;7:373. doi: 10.3389/fcimb.2017.00373. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28890882 Free PMC article. Review.
Cited by
- AMPK: opposing the metabolic changes in both tumour cells and inflammatory cells?
Dandapani M, Hardie DG. Dandapani M, et al. Biochem Soc Trans. 2013 Apr;41(2):687-93. doi: 10.1042/BST20120351. Biochem Soc Trans. 2013. PMID: 23514177 Free PMC article. Review. - Lipopolysaccharide promotes Drp1-dependent mitochondrial fission and associated inflammatory responses in macrophages.
Kapetanovic R, Afroz SF, Ramnath D, Lawrence GM, Okada T, Curson JE, de Bruin J, Fairlie DP, Schroder K, St John JC, Blumenthal A, Sweet MJ. Kapetanovic R, et al. Immunol Cell Biol. 2020 Aug;98(7):528-539. doi: 10.1111/imcb.12363. Epub 2020 Jul 20. Immunol Cell Biol. 2020. PMID: 32686869 Free PMC article. - Pathways to Parkinsonism Redux: convergent pathobiological mechanisms in genetics of Parkinson's disease.
Kumaran R, Cookson MR. Kumaran R, et al. Hum Mol Genet. 2015 Oct 15;24(R1):R32-44. doi: 10.1093/hmg/ddv236. Epub 2015 Jun 22. Hum Mol Genet. 2015. PMID: 26101198 Free PMC article. Review. - The molecular mechanisms associated with the physiological responses to inflammation and oxidative stress in cardiovascular diseases.
Zhazykbayeva S, Pabel S, Mügge A, Sossalla S, Hamdani N. Zhazykbayeva S, et al. Biophys Rev. 2020 Aug;12(4):947-968. doi: 10.1007/s12551-020-00742-0. Epub 2020 Jul 21. Biophys Rev. 2020. PMID: 32691301 Free PMC article. Review. - Damage-Induced Calcium Signaling and Reactive Oxygen Species Mediate Macrophage Activation in Zebrafish.
Sipka T, Peroceschi R, Hassan-Abdi R, Groß M, Ellett F, Begon-Pescia C, Gonzalez C, Lutfalla G, Nguyen-Chi M. Sipka T, et al. Front Immunol. 2021 Mar 26;12:636585. doi: 10.3389/fimmu.2021.636585. eCollection 2021. Front Immunol. 2021. PMID: 33841419 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R37-AI33443/AI/NIAID NIH HHS/United States
- P01 ES011163/ES/NIEHS NIH HHS/United States
- R37 AI033443/AI/NIAID NIH HHS/United States
- NS-056206/NS/NINDS NIH HHS/United States
- R01 AI033443/AI/NIAID NIH HHS/United States
- R01 NS056206/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases