Small-molecule inhibitors of the PI3K signaling network - PubMed (original) (raw)
Review
Small-molecule inhibitors of the PI3K signaling network
Colleen R McNamara et al. Future Med Chem. 2011 Apr.
Abstract
The phosphoinositide 3-kinase (PI3K) signaling pathway controls a wide variety of cellular processes including cell death and survival, cell migration, protein synthesis and metabolism. Aberrant PI3K-dependent signaling, mediated by Akt kinase, has been implicated in many human diseases including cancer, inflammation, cardiovascular disease and metabolic diseases, making this pathway a principle target for drug development. In this article we will summarize the PI3K signaling network and discuss current strategies for pathway inhibition. We will also explore the importance and emerging relevance of Akt-independent PI3K signaling pathways and discuss attempts being made to harness these pathways by inhibiting the binding of a product of PI3K, phosphatidylinositol-(3,4,5)-trisphosphate, to effector pleckstrin homology domains.
Figures
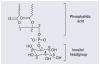
Figure 1. Phosphatidylinositol
An inositol headgroup is bound to phosphatidic acid via its 1′-hydroxyl group. Phosphatidic acid is composed of a glycerol backbone with fatty acids bound to carbons-1 and -2 (represented by waves in this graphic) and a phosphate group bound to carbon-3. The 4′- and 5′-hydroxyl groups are phosphorylated in PIP2. PI3K generates PIP3 by phosphorylating the 3′-hydroxyl group.
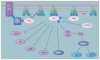
Figure 2. The phosphinositide-3-kinase/Akt signaling pathway
RTKs, activated in response to growth factor signaling, initiate PI3K signaling. Activated PI3K phosphorylates PIP2 to generate PIP3. Akt and PDK1 then bind to PIP3 via their PH domains and are localized to the plasma membrane. Akt is activated by phosphorylation of Thr-308 by PDK1 and Ser-473 by mTORC2. Activated Akt controls cell death and survival, cell cycle regulation, regulation of protein synthesis, angiogenesis and cell metabolism through activation or inhibition phosphorylations of many downstream substrates. Signaling is terminated when enzymes, such as PTEN, dephosphorylate PIP3. 4EBP1: Eukaryotic initiation factor 4E binding protein 1; Casp9: Caspase 9; FOXO: Forkhead family of transcription factor; GSK3: Glycogen synthase kinase 3; mTORC: Mammalian target of rapamycin (mTOR) complex; P: Phosphate; PDK1: Phosphoinositide-dependent kinase 1; PI3K: Phosphinositide-3-kinase; PIP2: Phosphatidylinositol-4–5-P2; PIP3: Phosphatidylinositol-3–4–5-P3; PTEN: Phosphatase and tensin homolog; Rheb: Ras homolog enriched in brain; RTK: Receptor tyrosine kinase; S6K1: p70S6 kinase; TSC: Tuberous sclerosis complex.
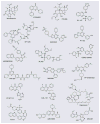
Figure 3
Representative PI3K pathway inhibitors.
Figure 4
Representative lipid-based antagonists of PIP3–PH domain interactions.
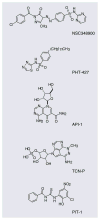
Figure 5
Representative nonlipid antagonists of PIP3–PH domain interactions.
Similar articles
- The PI3K/Akt/PTEN/mTOR pathway: a fruitful target for inducing cell death in rheumatoid arthritis?
Malemud CJ. Malemud CJ. Future Med Chem. 2015;7(9):1137-47. doi: 10.4155/fmc.15.55. Future Med Chem. 2015. PMID: 26132523 Review. - Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes.
Liu L, Zhao X, Pierre SV, Askari A. Liu L, et al. Am J Physiol Cell Physiol. 2007 Nov;293(5):C1489-97. doi: 10.1152/ajpcell.00158.2007. Epub 2007 Aug 29. Am J Physiol Cell Physiol. 2007. PMID: 17728397 - Novel PI3K/Akt inhibitors screened by the cytoprotective function of human immunodeficiency virus type 1 Tat.
Kim Y, Hollenbaugh JA, Kim DH, Kim B. Kim Y, et al. PLoS One. 2011;6(7):e21781. doi: 10.1371/journal.pone.0021781. Epub 2011 Jul 12. PLoS One. 2011. PMID: 21765914 Free PMC article. - Regulation of PI3K effector signalling in cancer by the phosphoinositide phosphatases.
Rodgers SJ, Ferguson DT, Mitchell CA, Ooms LM. Rodgers SJ, et al. Biosci Rep. 2017 Feb 10;37(1):BSR20160432. doi: 10.1042/BSR20160432. Print 2017 Feb 28. Biosci Rep. 2017. PMID: 28082369 Free PMC article. Review.
Cited by
- A small molecule activator of AKT does not reduce ischemic injury of the rat heart.
Moreira JB, Wohlwend M, Alves MN, Wisløff U, Bye A. Moreira JB, et al. J Transl Med. 2015 Mar 1;13:76. doi: 10.1186/s12967-015-0444-x. J Transl Med. 2015. PMID: 25889299 Free PMC article. - G12/13 signaling in asthma.
McDuffie EL, Panettieri RA Jr, Scott CP. McDuffie EL, et al. Respir Res. 2024 Aug 2;25(1):295. doi: 10.1186/s12931-024-02920-0. Respir Res. 2024. PMID: 39095798 Free PMC article. Review. - Identification of Inhibitory Compounds Against Singapore Grouper Iridovirus Infection by Cell Viability-Based Screening Assay and Droplet Digital PCR.
Jia K, Yuan Y, Liu W, Liu L, Qin Q, Yi M. Jia K, et al. Mar Biotechnol (NY). 2018 Feb;20(1):35-44. doi: 10.1007/s10126-017-9785-1. Epub 2017 Dec 5. Mar Biotechnol (NY). 2018. PMID: 29209860 - Endocytosis of Wingless via a dynamin-independent pathway is necessary for signaling in Drosophila wing discs.
Hemalatha A, Prabhakara C, Mayor S. Hemalatha A, et al. Proc Natl Acad Sci U S A. 2016 Nov 8;113(45):E6993-E7002. doi: 10.1073/pnas.1610565113. Epub 2016 Oct 25. Proc Natl Acad Sci U S A. 2016. PMID: 27791132 Free PMC article. - Src kinases in chondrosarcoma chemoresistance and migration: dasatinib sensitises to doxorubicin in TP53 mutant cells.
van Oosterwijk JG, van Ruler MA, Briaire-de Bruijn IH, Herpers B, Gelderblom H, van de Water B, Bovée JV. van Oosterwijk JG, et al. Br J Cancer. 2013 Sep 3;109(5):1214-22. doi: 10.1038/bjc.2013.451. Epub 2013 Aug 6. Br J Cancer. 2013. PMID: 23922104 Free PMC article.
References
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–619. - PubMed
- Martin TF. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu Rev Cell Dev Biol. 1998;14:231–264. - PubMed
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. - PubMed
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329–341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources