A CK2-dependent mechanism for activation of the JAK-STAT signaling pathway - PubMed (original) (raw)
A CK2-dependent mechanism for activation of the JAK-STAT signaling pathway
Ying Zheng et al. Blood. 2011.
Abstract
JAK-STAT signaling is involved in the regulation of cell survival, proliferation, and differentiation. JAK tyrosine kinases can be transiently activated by cytokines or growth factors in normal cells, whereas they become constitutively activated as a result of mutations that affect their function in tumors. Specifically, the JAK2V617F mutation is present in the majority of patients with myeloproliferative disorders (MPDs) and is implicated in the pathogenesis of these diseases. In the present study, we report that the kinase CK2 is a novel interaction partner of JAKs and is essential for JAK-STAT activation. We demonstrate that cytokine-induced activation of JAKs and STATs and the expression of suppressor of cytokine signaling 3 (SOCS-3), a downstream target, are inhibited by CK2 small interfering RNAs or pharmacologic inhibitors. Endogenous CK2 is associated with JAK2 and JAK1 and phosphorylates JAK2 in vitro. To extend these findings, we demonstrate that CK2 interacts with JAK2V617F and that CK2 inhibitors suppress JAK2V617F autophosphorylation and downstream signaling in HEL92.1.7 cells (HEL) and primary cells from polycythemia vera (PV) patients. Furthermore, CK2 inhibitors potently induce apoptosis of HEL cells and PV cells. Our data provide evidence for novel cross-talk between CK2 and JAK-STAT signaling, with implications for therapeutic intervention in JAK2V617F-positive MPDs.
Figures
Figure 1
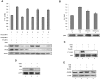
CK2 is required for OSM-induced STAT activation and gene expression. (A-E) Cell lysates were immunoblotted with the indicated antibodies. (A) MEFs were transfected with nontarget (NT) siRNA (100nM), CK2α siRNA (100nM), CK2β siRNA (100nM), or CK2α (50nM) plus CK2β siRNA (50nM) for 48 hours, then stimulated with 1 ng/mL of OSM for 10 minutes. The densitometric ratios of p-Y-STAT-3 versus STAT-3 were calculated. The values shown in lanes 4, 6, and 8 were compared with that of lane 2 (control, no inhibition) and the percentage of inhibition was determined. (B) MEFs were pretreated with 25-100μM TBB for 2 hours and then stimulated with 1 ng/mL of OSM for 10 minutes. The densitometric ratios of p-Y-STAT-3 versus STAT-3 were calculated. The values shown in lanes 3, 4, and 5 were compared with that of lane 2 (control, no inhibition) and the percentage of inhibition was determined. (C) MEFs were pretreated with TBB (50μM) for 2 and 4 hours and then stimulated with 0.1 ng/mL of OSM for 30 minutes. (D) MEFs were transfected with HA-CK2α or CK2α-inhibitor–resistant constructs for 24 hours, pretreated with TBB (50μM) for 2 hours and then stimulated with 1 ng/mL of OSM for 30 minutes. (E) MEFs were pretreated with CK2 inhibitors for 2 hours and then treated with OSM (10 ng/mL) for 30 minutes. (F) MEFs were transfected with nontarget (NT) siRNA (100nM), CK2α siRNA (100nM), CK2β siRNA (100nM), or CK2α (50nM) plus CK2β siRNA (50nM) for 48 hours, then stimulated with 0.1 ng/mL of OSM for 30 minutes. RNA was prepared and analyzed by RT-PCR for expression of SOCS-3 and GAPDH. The densitometric ratios of SOCS-3 versus GAPDH were calculated. The values of lanes 4, 6, and 8 were compared with that of lane 2 (control, no inhibition) and the percentage of inhibition was determined. (G) MEFs were pretreated with TBB (50μM) for 2 hours and then stimulated with different concentrations of OSM (0.1-0.5 ng/mL). Total mRNA was extracted and analyzed by RPA with probes specific to SOCS-3 and GAPDH (loading control). The densitometric ratios of SOCS-3 versus GAPDH were calculated. The value of lane 4 was compared with that of lane 3 (control, no inhibition), the value of lane 6 was compared with that of lane 5, and the percentage of inhibition was determined.
Figure 2
Inhibition of OSM-induced STAT-3 activation in human solid tumor cell lines by CK2 inhibitors. (A-C) Cell lysates were immunoblotted with the indicated antibodies. (A) CH235 human astroglioma cells were pretreated with TBB (50μM) for 2 hours and then stimulated with different concentrations of human OSM (0.5 and 1 ng/mL) for 30 minutes. The densitometric ratios of p-Y-STAT-3 versus STAT-3 were calculated. The value of lane 4 was compared with that of lane 3 (control, no inhibition), the value of lane 6 was compared with that of lane 5, and the percentage of inhibition was determined. γ2A-JAK2 human fibrosarcoma cells (B) and MDA-MB-231 human breast cancer cells (C) were pretreated with TBB (50μM) or emodin (50μM) for 2 hours and then stimulated with 0.5 ng/mL of human OSM for 30 minutes. The densitometric ratios of P-Y-STAT-3 versus STAT-3 were calculated. The values of lanes 3 and 4 were compared with that of lane 2 (control, no inhibition) and the percentage of inhibition was determined.
Figure 3
Inhibition of IFN-γ and GH signaling pathways by CK2 inhibitors. (A-C) Cell lysates were immunoblotted with the indicated antibodies. (A) MEFs were pretreated with TBB (50μM) or emodin (50μM) for 2 hours and then stimulated with 5 ng/mL of IFN-γ for 30 minutes. The densitometric ratios of P-Y-STAT-1 versus STAT-1 were calculated. The values of lanes 3 and 4 were compared with that of lane 2 (control, no inhibition) and the percentage of inhibition was determined. (B) MEFs were pretreated with TBB (50μM) or emodin (50μM) for 2 hours and then stimulated with 5 ng/mL of IFN-γ for 30 minutes. (C) γ2A-GHR-JAK2 cells were pretreated with TBB (50μM) or emodin (50μM) for 2 hours and then stimulated with 250 ng/mL of GH for 10 minutes. The densitometric ratios of P-Y-STAT-5 versus ACTIN were calculated. The values of lanes 3 and 4 were compared with that of lane 2 (control, no inhibition) and the percentage of inhibition was determined.
Figure 4
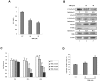
CK2 is required for OSM-induced JAK2 and JAK1 activation. (A) MEFs were transfected with nontarget (NT) siRNA (100nM), CK2α siRNA (100nM), CK2β siRNA (100nM), or CK2α (50nM) plus CK2β siRNA (50nM) for 48 hours and then stimulated with 5 ng/mL of OSM for 10 minutes. (B-C) MEFs were pretreated with TBB (50μM) or emodin (50μM) for 2 hours and then stimulated with 5 ng/mL OSM for 10 minutes. (A-B) The protein concentration of the cell lysates was measured in duplicate and 65 μg of total protein was analyzed for JAK2 pYpY 1007/1008 expression by ELISA and normalized to JAK2 expression, which is not affected by CK2 siRNAs. The value of the untreated sample was arbitrarily set as 1. Three independent experiments were performed and error bars show ± SD. *P < .05. Cell lysates were immunoblotted with the indicated antibodies (lower panels). (C) Cell lysates were blotted with the indicated antibodies. (D) MEFs were pretreated with TBB (50μM) for 2 hours and then stimulated with 10 ng/mL of OSM for 30 minutes. Lysates were immunoprecipitated with anti-gp130 antibody and analyzed by immunoblotting. The blot was detected with anti-phosphotyrosine antibody and then reprobed with gp130 antibody after stripping. (E) MEFs were pretreated with TBB (50μM) or emodin (50μM) for 2 hours and then stimulated with 0.1 ng/mL of OSM for 30 minutes. Cell lysates were immunoblotted with the indicated antibodies.
Figure 5
Co-IP of endogenous CK2 with JAK1 and JAK2 and phosphorylation of JAK2 by CK2 in vitro. Lysates of γ2A-JAK2 cells were immunoprecipitated (IP) with anti-CK2α and normal goat IgG (G-IgG, negative control) (A, E, H) or with anti-STAT-3, anti-JAK1, or normal rabbit IgG (R-IgG, negative control) (F-G). (B) γ2A-JAK2 cells were untreated or treated with 5 ng/mL of OSM for 15 minutes. Cell lysates were immunoprecipitated with the indicated antibodies. (C) Lysates of γ2A-GHR-JAK2 cells (lane 2) and γ2A-GHR-JAK2KD cells (lanes 1 and 3) were immunoprecipitated with the indicated antibodies. (D) Left panel: 293T cells were transfected with HA-tagged JAK2 full-length or deletion constructs, immunoprecipitated, and immunoblotted (IB) with the indicated antibodies. Right panel: schematic presentation of the proteins encoded by JAK2 constructs. The deleted amino acids are indicated. (I) 0.5 μg of JAK2 was incubated with 500 U of CK2 (α and β) in the absence or presence of 50μM of TBB and resolved by 6% SDS-PAGE, followed by autoradiography (top panel) and Coomassie blue staining (bottom panel).
Figure 6
Inhibition of autonomous activation of JAK-STAT signaling and induction of apoptosis in HEL cells by the CK2 inhibitor TBB. (A) Lysates of HEL cells were immunoprecipitated with anti-JAK2 and R-IgG and then immunoblotted with the indicated antibodies. (B) HEL cells were treated with different concentrations of TBB (10-50μM) for 4 hours. The protein concentration of cell lysates was measured in duplicate, and 65 μg of total protein was analyzed for JAK2 pYpY 1007/1008 expression with ELISA, and then normalized to JAK2 expression. The value of the untreated sample was arbitrarily set as 1. Three independent experiments were performed and error bars show ± SD. *P < .05. (C) HEL cells were treated with different concentrations of TBB (10-50μM) for 4 hours. Cell lysates were immunoblotted with the indicated antibodies. (D-F) HEL cells were treated with different concentrations of TBB (5-25μM) for 24 hours. (D) Cells were stained with annexin V and propidium iodide and examined by flow cytometry. Experiments were performed in triplicate and error bars show ± SD. *P < .05. (E) HEL cells were fixed overnight, stained with propidium iodide, and digested with RNase. The percentage of cells in the sub-G1, G1, S, and G2/M phases was examined by flow cytometry. (F) Cell lysates were immunoblotted with the indicated antibodies.
Figure 7
Inhibition of autonomous activation of JAK-STAT signaling and induction of apoptosis in primary PV cells by the CK2 inhibitor TBB. (A) Primary PV cells were treated with 10 or 50μM of TBB for 4 hours. The protein concentration of cell lysates was measured in duplicate and 100 μg of total protein was analyzed for JAK2 pYpY 1007/1008 expression with ELISA and then normalized to JAK2 expression. The value of the untreated sample was arbitrarily set as 1. Three independent experiments were performed and error bars show ± SD. *P < .05. (B) Primary PV cells were treated with 10 or 50μM TBB for 4 hours. Cell lysates were immunoblotted with the indicated antibodies. The densitometric ratios of P-Y-STAT-3 versus STAT-3, P-Y-STAT-5 versus STAT-5, and P-ERK versus ERK were calculated. The values of lanes 2 and 3 were compared with that of lane 1 (control, no inhibition) and the percentage of inhibition was determined. (C) Primary PV cells were treated with different concentrations of TBB (10-50μM) for the indicated times and cell survival was measured with the WST-1 assay. The value of the untreated sample (8 hours) was arbitrarily set as 1. Experiments were performed in triplicate and error bars show ± SD. *P < .05. (D) Primary PV cells were treated with 10 or 50μM TBB for 24 hours. Cells were stained with annexin V and propidium iodide and examined by flow cytometry. Experiments were performed in triplicate and error bars show ± SD. *P < .05.
Comment in
- JAKs, Stats, and CK2?
Carter-Su C, Argetsinger LS. Carter-Su C, et al. Blood. 2011 Jul 7;118(1):5-6. doi: 10.1182/blood-2011-05-352542. Blood. 2011. PMID: 21737607 No abstract available.
Similar articles
- CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients.
Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A. Pardanani A, et al. Leukemia. 2009 Aug;23(8):1441-5. doi: 10.1038/leu.2009.50. Epub 2009 Mar 19. Leukemia. 2009. PMID: 19295546 - Combined inhibition of Janus kinase 1/2 for the treatment of JAK2V617F-driven neoplasms: selective effects on mutant cells and improvements in measures of disease severity.
Liu PC, Caulder E, Li J, Waeltz P, Margulis A, Wynn R, Becker-Pasha M, Li Y, Crowgey E, Hollis G, Haley P, Sparks RB, Combs AP, Rodgers JD, Burn TC, Vaddi K, Fridman JS. Liu PC, et al. Clin Cancer Res. 2009 Nov 15;15(22):6891-900. doi: 10.1158/1078-0432.CCR-09-1298. Epub 2009 Nov 3. Clin Cancer Res. 2009. PMID: 19887489 - JAKs, Stats, and CK2?
Carter-Su C, Argetsinger LS. Carter-Su C, et al. Blood. 2011 Jul 7;118(1):5-6. doi: 10.1182/blood-2011-05-352542. Blood. 2011. PMID: 21737607 No abstract available. - JAK/STAT signal transduction: regulators and implication in hematological malignancies.
Valentino L, Pierre J. Valentino L, et al. Biochem Pharmacol. 2006 Mar 14;71(6):713-21. doi: 10.1016/j.bcp.2005.12.017. Epub 2006 Jan 19. Biochem Pharmacol. 2006. PMID: 16426581 Review. - The Development and Use of Janus Kinase 2 Inhibitors for the Treatment of Myeloproliferative Neoplasms.
Hobbs GS, Rozelle S, Mullally A. Hobbs GS, et al. Hematol Oncol Clin North Am. 2017 Aug;31(4):613-626. doi: 10.1016/j.hoc.2017.04.002. Epub 2017 May 17. Hematol Oncol Clin North Am. 2017. PMID: 28673391 Review.
Cited by
- Protein kinase CK2 in breast cancer: the CK2β regulatory subunit takes center stage in epithelial plasticity.
Filhol O, Giacosa S, Wallez Y, Cochet C. Filhol O, et al. Cell Mol Life Sci. 2015 Sep;72(17):3305-22. doi: 10.1007/s00018-015-1929-8. Epub 2015 May 20. Cell Mol Life Sci. 2015. PMID: 25990538 Free PMC article. Review. - Identification of a novel function of the clathrin-coated structure at the plasma membrane in facilitating GM-CSF receptor-mediated activation of JAK2.
Chen PH, Chien FC, Lee SP, Chan WE, Lin IH, Liu CS, Lee FJ, Lai JS, Chen P, Yang-Yen HF, Yen JJ. Chen PH, et al. Cell Cycle. 2012 Oct 1;11(19):3611-26. doi: 10.4161/cc.21920. Epub 2012 Aug 30. Cell Cycle. 2012. PMID: 22935703 Free PMC article. - Clinical-Grade Peptide-Based Inhibition of CK2 Blocks Viability and Proliferation of T-ALL Cells and Counteracts IL-7 Stimulation and Stromal Support.
Perera Y, Melão A, Ramón AC, Vázquez D, Ribeiro D, Perea SE, Barata JT. Perera Y, et al. Cancers (Basel). 2020 May 27;12(6):1377. doi: 10.3390/cancers12061377. Cancers (Basel). 2020. PMID: 32471246 Free PMC article. - Past, Present, and a Glance into the Future of Multiple Myeloma Treatment.
Elbezanti WO, Challagundla KB, Jonnalagadda SC, Budak-Alpdogan T, Pandey MK. Elbezanti WO, et al. Pharmaceuticals (Basel). 2023 Mar 8;16(3):415. doi: 10.3390/ph16030415. Pharmaceuticals (Basel). 2023. PMID: 36986514 Free PMC article. Review. - Cardiotrophin 1 stimulates beneficial myogenic and vascular remodeling of the heart.
Abdul-Ghani M, Suen C, Jiang B, Deng Y, Weldrick JJ, Putinski C, Brunette S, Fernando P, Lee TT, Flynn P, Leenen FHH, Burgon PG, Stewart DJ, Megeney LA. Abdul-Ghani M, et al. Cell Res. 2017 Oct;27(10):1195-1215. doi: 10.1038/cr.2017.87. Epub 2017 Aug 8. Cell Res. 2017. PMID: 28785017 Free PMC article.
References
- Chen S-H, Benveniste EN. Oncostatin M: a pleiotropic cytokine in the central nervous system. Cytokine Growth Factor Rev. 2004;15(5):379–391. - PubMed
- Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3(11):900–911. - PubMed
- Parganas E, Wang D, Stravopodis D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93(3):385–395. - PubMed
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS-54158/NS/NINDS NIH HHS/United States
- R01 NS045290/NS/NINDS NIH HHS/United States
- P30 AT050948/AT/NCCIH NIH HHS/United States
- R21 NS054158/NS/NINDS NIH HHS/United States
- R21 NS066332/NS/NINDS NIH HHS/United States
- R56 DK046395/DK/NIDDK NIH HHS/United States
- NS-36765/NS/NINDS NIH HHS/United States
- NS-45290/NS/NINDS NIH HHS/United States
- P30 DK079337/DK/NIDDK NIH HHS/United States
- S10 RR-2782/RR/NCRR NIH HHS/United States
- R01 NS036765/NS/NINDS NIH HHS/United States
- S10 RR027822/RR/NCRR NIH HHS/United States
- DK-46395/DK/NIDDK NIH HHS/United States
- GM-65959/GM/NIGMS NIH HHS/United States
- R01 DK046395/DK/NIDDK NIH HHS/United States
- NS-66332/NS/NINDS NIH HHS/United States
- U54 CA100949/CA/NCI NIH HHS/United States
- P30 AR050948/AR/NIAMS NIH HHS/United States
- R01 GM065959/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous