Nicotine behavioral pharmacology: clues from planarians - PubMed (original) (raw)
Nicotine behavioral pharmacology: clues from planarians
Scott M Rawls et al. Drug Alcohol Depend. 2011.
Abstract
Background: Nicotine is one of the world's most addictive substances and the primary reason that humans inhale tobacco smoke. The pharmacological effects of nicotine can be investigated in planarians, aquatic flatworms that possess an integrated neural network including cephalic ganglia that some consider the earliest 'brain' and spinal cord. Here, we tested the hypothesis that nicotine exposure elicits mammalian-like behaviors in planarians.
Methods: Planarian motility and stereotypy (C-shape hyperkinesias) were quantified following acute nicotine exposure. During repeated nicotine exposure, we investigated the presence of withdrawal, tolerance, behavioral sensitization, and environmental place conditioning.
Results: Acute nicotine exposure increased stereotypical activity and elicited biphasic effects on motility. A low concentration (0.01 mM) increased motility whereas higher concentrations (0.3-10mM) elicited the opposite effect. Planarians exposed to nicotine (0.03 mM) for 60 min and then tested in water displayed reduced motility that was not observed during exposure to water, acute nicotine, or continuous nicotine. Nicotine-treated planarians withdrawn from the drug for 3 days before being challenged with nicotine displayed behavioral sensitization at low concentrations (0.1, 0.3mM) but tolerance at higher concentrations (1, 3mM). Planarians conditioned with nicotine in the ambient light (non-preferred environment) displayed a reduction in their natural preference for a dark environment.
Conclusions: The present results suggest nicotine elicits mammalian-like effects in planarians, including decreased motility and increased stereotypy following acute administration and abstinence-induced withdrawal, behavioral sensitization, tolerance, and place conditioning during repeated exposure.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Figures
Fig. 1
Acute nicotine administration increases planarian motility and stereotypy. Planarians were exposed to different concentrations of nicotine (0.01, 0.03, 0.1, 0.3, 1, 3, 5, 10 mM). Motility and stereotypical activity was quantified during 5 min of nicotine exposure and presented as mean activity counts + S.E.M in 5 min. **p < 0.01 compared to water control for stereotypical activity and ++p < 0.01 compared to water control for motility. N = 8 planarians per group.
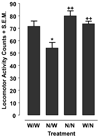
Fig. 2
Nicotine (0.03 mM) produces abstinence-induced withdrawal behavior in planarians. Planarians pre-treated with nicotine (N) or water (W) for 60 min were then tested in N or water (W) for 5 min. Data are presented as mean motility counts + S.E.M in 5 min. *p < 0.05 compared to W/W and ++p < 0.01 compared to N/W. N = 8 planarians per group.
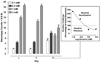
Fig. 3
Low concentrations of nicotine produce sensitization of stereotypical activity and high concentrations of nicotine produce tolerance to stereotypical activity. Planarians were exposed to nicotine (0.1, 0.3, 1, 3 mM) twice on day 1 (120 min apart) and then re-exposed to the same concentration of nicotine for 5 min on day 4. Data are expressed as mean stereotypy counts + S.E.M. during 5 min of nicotine exposure versus day (1, 1’, 4). **p < 0.01 compared to the stereotypy counts produced by initial drug exposure (day 1). N = 8 planarians per group. Box) The percentage of the initial nicotine response (day 1) produced by nicotine challenge on day 4 is plotted versus log nicotine concentration.
Fig. 4
Nicotine elicits environmental place conditioning in planarians. Table) The conditioning phase paired nicotine (N) (0.1 mM) or water (W) with the ambient light (L) or dark (D) for 10 min. Preference testing was conducted 2 h later when planarians were placed for 10 min at the center of a petri dish containing water (half of dish was covered on the top and bottom by paper to create a dark side and ambient light side). Data are presented as the (A) number of planarians (out of 20) that spent a greater amount of time in the dark and (B) mean preference score (s) + S.E.M. **p < 0.01, *p < 0.05 compared to W(L)/W(D). N = 20 planarians per group.
Similar articles
- Mu Opioid Receptor Agonist DAMGO Produces Place Conditioning, Abstinence-induced Withdrawal, and Naltrexone-Dependent Locomotor Activation in Planarians.
Dziedowiec E, Nayak SU, Gruver KS, Jennings T, Tallarida CS, Rawls SM. Dziedowiec E, et al. Neuroscience. 2018 Aug 21;386:214-222. doi: 10.1016/j.neuroscience.2018.06.029. Epub 2018 Jun 27. Neuroscience. 2018. PMID: 29958944 Free PMC article. - Nicotine chronic tolerance development and withdrawal in the planaria (Schmidtea mediterranea).
Sal F, Prados J, Urcelay GP. Sal F, et al. Pharmacol Biochem Behav. 2021 Jan;200:173075. doi: 10.1016/j.pbb.2020.173075. Epub 2020 Nov 24. Pharmacol Biochem Behav. 2021. PMID: 33245983 - Cotinine antagonizes the behavioral effects of nicotine exposure in the planarian Girardia tigrina.
Bach DJ, Tenaglia M, Baker DL, Deats S, Montgomery E, Pagán OR. Bach DJ, et al. Neurosci Lett. 2016 Oct 6;632:204-8. doi: 10.1016/j.neulet.2016.09.005. Epub 2016 Sep 8. Neurosci Lett. 2016. PMID: 27616704 Free PMC article. - Cotinine influences the effect of high and low nicotine concentrations on planarian motility differently.
Ruble M, Simpson N, Smith B, Adeshina W, Snyder E, Pagán OR. Ruble M, et al. Neurosci Lett. 2024 Oct 15;841:137955. doi: 10.1016/j.neulet.2024.137955. Epub 2024 Aug 28. Neurosci Lett. 2024. PMID: 39214334 - A cembranoid from tobacco prevents the expression of nicotine-induced withdrawal behavior in planarian worms.
Pagán OR, Rowlands AL, Fattore AL, Coudron T, Urban KR, Bidja AH, Eterović VA. Pagán OR, et al. Eur J Pharmacol. 2009 Aug 1;615(1-3):118-24. doi: 10.1016/j.ejphar.2009.05.022. Epub 2009 May 30. Eur J Pharmacol. 2009. PMID: 19490913 Free PMC article.
Cited by
- Mu Opioid Receptor Agonist DAMGO Produces Place Conditioning, Abstinence-induced Withdrawal, and Naltrexone-Dependent Locomotor Activation in Planarians.
Dziedowiec E, Nayak SU, Gruver KS, Jennings T, Tallarida CS, Rawls SM. Dziedowiec E, et al. Neuroscience. 2018 Aug 21;386:214-222. doi: 10.1016/j.neuroscience.2018.06.029. Epub 2018 Jun 27. Neuroscience. 2018. PMID: 29958944 Free PMC article. - Energy drink produces aversive effects in planarians.
Mokkarala P, Shekarabi A, Wiah S, Rawls SM. Mokkarala P, et al. Physiol Behav. 2022 Oct 15;255:113933. doi: 10.1016/j.physbeh.2022.113933. Epub 2022 Jul 26. Physiol Behav. 2022. PMID: 35905805 Free PMC article. - Evaluating a school-based science program that teaches the physiological effects of nicotine.
Cameron JL, Brasch K, Strong D, Paul B, Cavanaugh E, Thakur S, Watson MN, Jennings T, Nayak SU, Rawls SM. Cameron JL, et al. Addict Behav. 2021 Mar;114:106744. doi: 10.1016/j.addbeh.2020.106744. Epub 2020 Nov 28. Addict Behav. 2021. PMID: 33291057 Free PMC article. - Schild Analysis of the Interaction between Parthenolide and Cocaine Suggests an Allosteric Relationship for Their Effects on Planarian Motility.
Kakuturu J, O'Brien M, Pagán OR. Kakuturu J, et al. Biomolecules. 2024 Sep 18;14(9):1168. doi: 10.3390/biom14091168. Biomolecules. 2024. PMID: 39334934 Free PMC article. - Ethanol and cocaine: environmental place conditioning, stereotypy, and synergism in planarians.
Tallarida CS, Bires K, Avershal J, Tallarida RJ, Seo S, Rawls SM. Tallarida CS, et al. Alcohol. 2014 Sep;48(6):579-86. doi: 10.1016/j.alcohol.2014.07.006. Epub 2014 Aug 7. Alcohol. 2014. PMID: 25212751 Free PMC article.
References
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch. Gen. Psychiatry. 2006;63:1386–1395. - PubMed
- Buttarelli FR, Pontieri FE, Margotta V, Palladini G. Acetylcholine/dopamine interaction in planaria. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2000;125:225–231. - PubMed
- Chae Y, Yeom M, Han JH, Park HJ, Hahm DH, Shim I, Lee HS, Lee H. Effect of acupuncture on anxiety-like behavior during nicotine withdrawal and relevant mechanisms. Neurosci. Lett. 2008;430:98–102. - PubMed
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–362. - PubMed
- Domino EF. Nicotine induced behavioral locomotor sensitization. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2001;25:59–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources