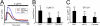Mutagenesis mapping of the presenilin 1 calcium leak conductance pore - PubMed (original) (raw)
Mutagenesis mapping of the presenilin 1 calcium leak conductance pore
Omar Nelson et al. J Biol Chem. 2011.
Abstract
Missense mutations in presenilin 1 (PS1) and presenilin 2 (PS2) proteins are a major cause of familial Alzheimer disease. Presenilins are proteins with nine transmembrane (TM) domains that function as catalytic subunits of the γ-secretase complex responsible for the cleavage of the amyloid precursor protein and other type I transmembrane proteins. The water-filled cavity within presenilin is necessary to mediate the intramembrane proteolysis reaction. Consistent with this idea, cysteine-scanning mutagenesis and NMR studies revealed a number of water-accessible residues within TM7 and TM9 of mouse PS1. In addition to γ-secretase function, presenilins also demonstrate a low conductance endoplasmic reticulum Ca(2+) leak function, and many familial Alzheimer disease presenilin mutations impair this function. To map the potential Ca(2+) conductance pore in PS1, we systematically evaluated endoplasmic reticulum Ca(2+) leak activity supported by a series of cysteine point mutants in TM6, TM7, and TM9 of mouse PS1. The results indicate that TM7 and TM9, but not TM6, could play an important role in forming the conductance pore of PS1. These results are consistent with previous cysteine-scanning mutagenesis and NMR analyses of PS1 and provide further support for our hypothesis that the hydrophilic catalytic cavity of presenilins may also constitute a Ca(2+) conductance pore.
Figures
FIGURE 1.
Cysteine-less mPS1 rescues the ER Ca2+ leak pathway in stably transfected DKO cells. A, shown are the IO-induced Ca2+ signal curves in DKO cells (blue line) and in DKO cells stably transfected with WT mPS1 (black line) and Cys-less mPS1 (red line) constructs. B, the average size of the IO-releasable Ca2+ pool is shown for DKO cells and for DKO cells stably transfected with mPS1 and Cys-less mPS1 constructs as the mean ± S.D. (n = number of cells analyzed). Compared with DKO MEFs, the size of the IO-releasable Ca2+ pool is significantly smaller (***, p < 0.05 by analysis of variance (ANOVA)) in Cys-less mPS1 and WT mPS1. C, the average ER Ca2+ concentrations ([Ca2+]ER) are shown for DKO MEFs and DKO MEFs stably expressing WT mPS1 and Cys-less mPS1 cells as the mean ± S.D. (n = number of cells analyzed). Compared with DKO MEFs, the [Ca2+]ER level is significantly smaller (***, p < 0.05 by ANOVA) in Cys-less mPS1 and WT mPS1.
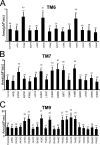
FIGURE 2.
Summary of the IO-releasable Ca2+ ER pool in mutants of TM6, TM7, and TM9 mPS1. A, the average size of the IO-releasable Ca2+ pool is shown for DKO cells stably expressing Cys mutants in TM6 as the mean ± S.D. (n = number of cells analyzed). Compared with cells stably transfected with Cys-less mPS1 and WT mPS1, the size of the IO-releasable Ca2+ pool is significantly larger (***, p < 0.05 by ANOVA) in T245C, S254, and A260C of TM6, whereas the other TM six residues did not shown any significant difference. B, the average size of the IO-releasable Ca2+ pool is shown for DKO cells stably expressing Cys mutants in TM7 as the mean ± S.D. (n = number of cells analyzed). Compared with cells stably transfected with Cys-less mPS1 and WT mPS1, the size of the IO-releasable Ca2+ pool is significantly larger (***, p < 0.05 by ANOVA) in E376C, G382C, G384C, D385C, I387C, and Y389C of TM7, whereas the other TM six residues did not shown any significant difference. C, the average size of the IO-releasable Ca2+ pool is shown for DKO cells stably expressing Cys mutants in TM9 as the mean ± S.D. (n = number of cells analyzed). Compared with cells stably transfected with Cys-less mPS1 and WT mPS1, the size of the IO-releasable Ca2+ pool is significantly larger (***, p < 0.05 by ANOVA) in L435C, P436C, I439C, F441C, F455C, Y446C, F447C, and T449C of TM9, whereas the other TM six residues did not shown any significant difference.
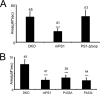
FIGURE 3.
The mPS1-Δloop does not affect the ER Ca2+ leak pathway in stably transfected DKO cells. A, the average size of the IO-releasable Ca2+ pool is shown for DKO MEFs and for DKO cells stably transfected with mPS1 and mPS1-Δloop constructs as the mean ± S.D. (n = number of cells analyzed). Compared with DKO MEFs, the size of the IO-releasable Ca2+ pool is significantly smaller (***, p < 0.05 by ANOVA) in WT mPS1-transfected cells; however, there is no significant difference between DKO MEFs and mPS1-Δloop. B, the average size of the IO-releasable Ca2+ pool is shown for DKO MEFs and for DKO cells stably transfected with WT mPS1 and mutant P433A and P433L constructs as the mean ± S.D. (n = number of cells analyzed). Compared with DKO MEFs, the size of the IO-releasable Ca2+ pool is significantly smaller (***, p < 0.05 by ANOVA) in WT mPS1-, P433A- and P433L-transfected cells.
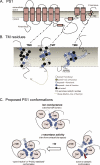
FIGURE 4.
Results of structure-function analysis of TM6, TM7, and TM9 of mPS1. A, vertical representation of PS1 TM1–TM9. The conformation of the TM7–TM9 region of PS1 is based on NMR studies (33). B, structure-function analysis of TM6, TM7, and TM9 of mPS1. Amino acids are represented by the single letter code. White residues are “buried” in SCAM experiments (29, 30) and do not cause loss of Ca2+ leak activity when mutated to cysteine (Group 1). Gray residues are buried in SCAM experiments and cause loss of Ca2+ leak activity when mutated to cysteine (Group 2). Light blue residues are exposed to water in SCAM experiments and do not cause loss of Ca2+ leak activity when mutated to cysteine (Group 3). Dark blue residues are exposed to water and cause loss of Ca2+ leak activity when mutated to cysteine (Group 4). Black residues were not tested in the Ca2+ assay (Group 5). C, comparison of PS1 TM6, TM7, and TM9 arrangement in the proposed Ca2+ channel or γ-secretase complex. Views from the ER lumen (for Ca2+ channel) and from the extracellular space (for γ-secretase) are shown.
Similar articles
- Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1.
Nelson O, Tu H, Lei T, Bentahir M, de Strooper B, Bezprozvanny I. Nelson O, et al. J Clin Invest. 2007 May;117(5):1230-9. doi: 10.1172/JCI30447. Epub 2007 Apr 12. J Clin Invest. 2007. PMID: 17431506 Free PMC article. - Thapsigargin affects presenilin-2 but not presenilin-1 regulation in SK-N-BE cells.
Rivabene R, Visentin S, Piscopo P, De Nuccio C, Crestini A, Svetoni F, Rosa P, Confaloni A. Rivabene R, et al. Exp Biol Med (Maywood). 2014 Feb;239(2):213-24. doi: 10.1177/1535370213514317. Epub 2013 Dec 20. Exp Biol Med (Maywood). 2014. PMID: 24363250 - Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations.
Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Tu H, et al. Cell. 2006 Sep 8;126(5):981-93. doi: 10.1016/j.cell.2006.06.059. Cell. 2006. PMID: 16959576 Free PMC article. - Presenilin 1 Regulates Membrane Homeostatic Pathways that are Dysregulated in Alzheimer's Disease.
Deaton CA, Johnson GVW. Deaton CA, et al. J Alzheimers Dis. 2020;77(3):961-977. doi: 10.3233/JAD-200598. J Alzheimers Dis. 2020. PMID: 32804090 Free PMC article. Review. - Presenilins: role in calcium homeostasis.
Honarnejad K, Herms J. Honarnejad K, et al. Int J Biochem Cell Biol. 2012 Nov;44(11):1983-6. doi: 10.1016/j.biocel.2012.07.019. Epub 2012 Jul 27. Int J Biochem Cell Biol. 2012. PMID: 22842534 Review.
Cited by
- Presenilins and calcium signaling-systems biology to the rescue.
Bezprozvanny I. Bezprozvanny I. Sci Signal. 2013 Jul 9;6(283):pe24. doi: 10.1126/scisignal.2004296. Sci Signal. 2013. PMID: 23838181 Free PMC article. Review. - Carvedilol suppresses ryanodine receptor-dependent Ca2+ bursts in human neurons bearing PSEN1 variants found in early onset Alzheimer's disease.
Hori A, Inaba H, Hato T, Tanaka K, Sato S, Okamoto M, Horiuchi Y, Paran FJ, Tabe Y, Mori S, Rosales C, Akamatsu W, Murayama T, Kurebayashi N, Sakurai T, Ai T, Miida T. Hori A, et al. PLoS One. 2024 Aug 22;19(8):e0291887. doi: 10.1371/journal.pone.0291887. eCollection 2024. PLoS One. 2024. PMID: 39173065 Free PMC article. - The very many faces of presenilins and the γ-secretase complex.
Smolarkiewicz M, Skrzypczak T, Wojtaszek P. Smolarkiewicz M, et al. Protoplasma. 2013 Oct;250(5):997-1011. doi: 10.1007/s00709-013-0494-y. Epub 2013 Mar 16. Protoplasma. 2013. PMID: 23504135 Free PMC article. Review. - Bridging Genomics to Phenomics at Atomic Resolution through Variation Spatial Profiling.
Wang C, Balch WE. Wang C, et al. Cell Rep. 2018 Aug 21;24(8):2013-2028.e6. doi: 10.1016/j.celrep.2018.07.059. Cell Rep. 2018. PMID: 30134164 Free PMC article. - Can the calcium hypothesis explain synaptic loss in Alzheimer's disease?
Popugaeva E, Bezprozvanny I. Popugaeva E, et al. Neurodegener Dis. 2014;13(2-3):139-41. doi: 10.1159/000354778. Epub 2013 Sep 24. Neurodegener Dis. 2014. PMID: 24080896 Free PMC article.
References
- Urban S., Schlieper D., Freeman M. (2002) Curr. Biol. 12, 1507–1512 - PubMed
- Brown M. S., Ye J., Rawson R. B., Goldstein J. L. (2000) Cell 100, 391–398 - PubMed
- Laudon H., Hansson E. M., Melén K., Bergman A., Farmery M. R., Winblad B., Lendahl U., von Heijne G., Näslund J. (2005) J. Biol. Chem. 280, 35352–35360 - PubMed
- Spasic D., Tolia A., Dillen K., Baert V., De Strooper B., Vrijens S., Annaert W. (2006) J. Biol. Chem. 281, 26569–26577 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous