Molecular definition of vaginal microbiota in East African commercial sex workers - PubMed (original) (raw)
. 2011 Jun;77(12):4066-74.
doi: 10.1128/AEM.02943-10. Epub 2011 Apr 29.
Matthew G Links, Janet E Hill, Tim J Dumonceaux, Joshua Kimani, Walter Jaoko, Charles Wachihi, Jane Njeri Mungai, Geoffrey A Peters, Shaun Tyler, Morag Graham, Alberto Severini, Keith R Fowke, T Blake Ball, Francis A Plummer
Affiliations
- PMID: 21531840
- PMCID: PMC3131651
- DOI: 10.1128/AEM.02943-10
Molecular definition of vaginal microbiota in East African commercial sex workers
John J Schellenberg et al. Appl Environ Microbiol. 2011 Jun.
Abstract
Resistance to HIV infection in a cohort of commercial sex workers living in Nairobi, Kenya, is linked to mucosal and antiinflammatory factors that may be influenced by the vaginal microbiota. Since bacterial vaginosis (BV), a polymicrobial dysbiosis characterized by low levels of protective Lactobacillus organisms, is an established risk factor for HIV infection, we investigated whether vaginal microbiology was associated with HIV-exposed seronegative (HESN) or HIV-seropositive (HIV(+)) status in this cohort. A subset of 44 individuals was selected for deep-sequencing analysis based on the chaperonin 60 (cpn60) universal target (UT), including HESN individuals (n = 16), other HIV-seronegative controls (HIV-N, n = 16), and HIV(+) individuals (n = 12). Our findings indicate exceptionally high phylogenetic resolution of the cpn60 UT using reads as short as 200 bp, with 54 species in 29 genera detected in this group. Contrary to our initial hypothesis, few differences between HESN and HIV-N women were observed. Several HIV(+) women had distinct profiles dominated by Escherichia coli. The deep-sequencing phylogenetic profile of the vaginal microbiota corresponds closely to BV(+) and BV(-) diagnoses by microscopy, elucidating BV at the molecular level. A cluster of samples with intermediate abundance of Lactobacillus and dominant Gardnerella was identified, defining a distinct BV phenotype that may represent a transitional stage between BV(+) and BV(-). Several alpha- and betaproteobacteria, including the recently described species Variovorax paradoxus, were found to correlate positively with increased Lactobacillus levels that define the BV(-) ("normal") phenotype. We conclude that cpn60 UT is ideally suited to next-generation sequencing technologies for further investigation of microbial community dynamics and mucosal immunity underlying HIV resistance in this cohort.
Figures
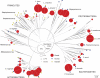
Fig. 1.
Phylogeny of 420 unique cpn60 UT sequences generated from vaginal specimens of African CSW by deep sequencing, clone library construction, and selective culture. Branches are collapsed into clusters for visual clarity (see Table S1 in the supplemental material). The relative abundance of deep-sequencing reads for each cluster is proportional to the area of the red circles, as indicated in the abundance scale. Blue and yellow circles indicate the presence of clones and culture-based isolates, respectively. Trimmed cpn60 UT sequences (200 bp, no internal stop codons) were aligned using Clustal in MEGA 4.0 and bootstrap values based on 500 replicates conducted using the neighbor-joining method. Branch segments proceeding from unsupported nodes (bootstrap value, <50%) are shown in gray. The scale bar indicates phylogenetic distance in base pair substitutions per site. Detailed abundance information and the species and strain designations for each branch and cluster are shown in Table S1 in the supplemental material.
Fig. 2.
Distribution of higher-order phylotypes in each of 44 individuals reveals patterns associated with BV diagnosis by Nugent score and HIV serostatus (A). Each pie chart represents a single sample, sorted from left to right by decreasing frequency of the total reads for all Lactobacillales OTUs. Abundance-weighted UniFrac PCA of 44 samples for BV diagnosis by Nugent score is shown (B to D). Groups: HESN (triangles), HIV-N (squares), HIV+ (circles); BV− (yellow), BVI (green), and BV+ (red). Reclassification of samples based on molecular pattern is also shown (C). Groups: BV− (yellow), mBVI (green), BV+ (red), and outliers (blue). PCA of group level phylogenies demonstrates that samples do not cluster by HESN or HIV+ status (D).
Fig. 3.
Heat map of deep-sequencing OTUs detected in at least 25% of the total study group members, organized in rows by HIV serostatus and BV status. OTUs are organized into columns by higher-order phylotype. The scale indicates the percentage of the total reads for each individual. Selected OTUs referred to in text are indicated at the bottom. A full listing of the OTUs, along with abundance information and statistical comparisons by HESN or HIV+ status and BV status are provided in Tables S4 and S5 in the supplemental material.
Fig. 4.
_k_-means clustering of OTUs using Genespring software reveals patterns associated with BV+ (clusters 1 and 2), mBVI (clusters 3 and 4), and BV− (clusters 5 and 6) status. A list of the OTUs associated with each cluster is shown in Table S6 in the supplemental material.
Similar articles
- Gardnerella vaginalis Subgroups Defined by cpn60 Sequencing and Sialidase Activity in Isolates from Canada, Belgium and Kenya.
Schellenberg JJ, Paramel Jayaprakash T, Withana Gamage N, Patterson MH, Vaneechoutte M, Hill JE. Schellenberg JJ, et al. PLoS One. 2016 Jan 11;11(1):e0146510. doi: 10.1371/journal.pone.0146510. eCollection 2016. PLoS One. 2016. PMID: 26751374 Free PMC article. - Selection, phenotyping and identification of acid and hydrogen peroxide producing bacteria from vaginal samples of Canadian and East African women.
Schellenberg JJ, Dumonceaux TJ, Hill JE, Kimani J, Jaoko W, Wachihi C, Mungai JN, Lane M, Fowke KR, Ball TB, Plummer FA. Schellenberg JJ, et al. PLoS One. 2012;7(7):e41217. doi: 10.1371/journal.pone.0041217. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22844440 Free PMC article. - The significance of Lactobacillus crispatus and L. vaginalis for vaginal health and the negative effect of recent sex: a cross-sectional descriptive study across groups of African women.
Jespers V, van de Wijgert J, Cools P, Verhelst R, Verstraelen H, Delany-Moretlwe S, Mwaura M, Ndayisaba GF, Mandaliya K, Menten J, Hardy L, Crucitti T; Vaginal Biomarkers Study Group. Jespers V, et al. BMC Infect Dis. 2015 Mar 4;15:115. doi: 10.1186/s12879-015-0825-z. BMC Infect Dis. 2015. PMID: 25879811 Free PMC article. - Cholesterol-Dependent Cytolysins Produced by Vaginal Bacteria: Certainties and Controversies.
Pleckaityte M. Pleckaityte M. Front Cell Infect Microbiol. 2020 Jan 10;9:452. doi: 10.3389/fcimb.2019.00452. eCollection 2019. Front Cell Infect Microbiol. 2020. PMID: 31998661 Free PMC article. Review. - Characterization of the vaginal microflora in health and disease.
Datcu R. Datcu R. Dan Med J. 2014 Apr;61(4):B4830. Dan Med J. 2014. PMID: 24814599 Review.
Cited by
- Biofilm-Forming Capacity and Drug Resistance of Different Gardnerella Subgroups Associated with Bacterial Vaginosis.
Qin H, Liu Y, Zhai Z, Xiao B. Qin H, et al. Microorganisms. 2023 Aug 30;11(9):2186. doi: 10.3390/microorganisms11092186. Microorganisms. 2023. PMID: 37764030 Free PMC article. - Crosstalk between the Resident Microbiota and the Immune Cells Regulates Female Genital Tract Health.
Santacroce L, Palmirotta R, Bottalico L, Charitos IA, Colella M, Topi S, Jirillo E. Santacroce L, et al. Life (Basel). 2023 Jul 9;13(7):1531. doi: 10.3390/life13071531. Life (Basel). 2023. PMID: 37511906 Free PMC article. Review. - Association of Pregnancy and HIV Status With Molecular-Bacterial Vaginosis in Indian Women.
Tuddenham S, Shafiq M, Mathad JS, Alexander M, Naik S, Kulkarni V, Deshpande P, Humphrys MS, Holm JB, Khan N, Yadana S, Cheedalla A, Bhosale R, Ghanem KG, Wang T, Wang S, Ma B, Ravel J, Gupta A, Shivakoti R. Tuddenham S, et al. J Acquir Immune Defic Syndr. 2023 Aug 15;93(5):422-430. doi: 10.1097/QAI.0000000000003215. J Acquir Immune Defic Syndr. 2023. PMID: 37155962 Free PMC article. - Association of key species of vaginal bacteria of recurrent bacterial vaginosis patients before and after oral metronidazole therapy with short- and long-term clinical outcomes.
Mollin A, Katta M, Sobel JD, Akins RA. Mollin A, et al. PLoS One. 2022 Jul 28;17(7):e0272012. doi: 10.1371/journal.pone.0272012. eCollection 2022. PLoS One. 2022. PMID: 35901180 Free PMC article. - Research Progress on the Correlation Between Gardnerella Typing and Bacterial Vaginosis.
Qin H, Xiao B. Qin H, et al. Front Cell Infect Microbiol. 2022 Mar 25;12:858155. doi: 10.3389/fcimb.2022.858155. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35402309 Free PMC article. Review.
References
- Ahmed N., et al. 2010. Suppression of human immunodeficiency virus type 1 replication in macrophages by commensal bacteria preferentially stimulating Toll-like receptor 4. J. Gen. Virol. 91:2804–2813 - PubMed
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 - PubMed
- Amsel R., et al. 1983. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 74:14–22 - PubMed
- Anesti V., et al. 2005. Isolation and molecular detection of methylotrophic bacteria occurring in the human mouth. Environ. Microbiol. 7:1227–1238 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials