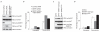WWP2 is an E3 ubiquitin ligase for PTEN - PubMed (original) (raw)
WWP2 is an E3 ubiquitin ligase for PTEN
Subbareddy Maddika et al. Nat Cell Biol. 2011 Jun.
Abstract
PTEN, a lipid phosphatase, is one of the most frequently mutated tumour suppressors in human cancer. Several recent studies have highlighted the importance of ubiquitylation in regulating PTEN tumour-suppressor function, but the enzymatic machinery required for PTEN ubiquitylation is not clear. In this study, by using a tandem affinity-purification approach, we have identified WWP2 (also known as atrophin-1-interacting protein 2, AIP-2) as a PTEN-interacting protein. WWP2 is an E3 ubiquitin ligase that belongs to the NEDD4-like protein family, which is involved in regulating transcription, embryonic stem-cell fate, cellular transport and T-cell activation processes. We show that WWP2 physically interacts with PTEN and mediates its degradation through a ubiquitylation-dependent pathway. Functionally, we show that WWP2 controls cellular apoptosis and is required for tumorigenicity of cells. Collectively, our results reveal a functional E3 ubiquitin ligase for PTEN that plays a vital role in tumour-cell survival.
Figures
Figure 1
WWP2 interacts with PTEN. (a) Immunoprecipitation (IP) using either control IgG or anti-PTEN antibody was carried out using extracts prepared from 293T cells. The endogenous interaction of WWP2, NEDD4-1 or EDD with PTEN was evaluated by immunoblotting (WB) with their respective antibodies. (b) Immunoprecipitation using control IgG or anti-Flag (PTEN) antibody was carried out using extracts prepared from either parental 293T cells or 293T derivative cells stably expressing Flag-tagged PTEN. The presence of WWP2 or NEDD4-1 in these immunoprecipitates was evaluated by immunoblotting with their respective antibodies. (c) GST pulldown assay was carried out using immobilized control GST or GST-PTEN fusion proteins on agarose beads followed by incubation with extracts prepared from 293T cells. The in vitro interaction of WWP2 with PTEN was assessed by immunoblotting with WWP2-specific antibodies. (d) Schematic representation of N-terminal Flag-tagged full-length PTEN (FL), along with its various deletion mutants (D1–D7). (e) 293T cells were co-transfected with the indicated Flag-tagged PTEN constructs along with those encoding Myc-tagged WWP2, and the interaction between PTEN and WWP2 was determined by immunoprecipitation and immunoblotting with the indicated antibodies. Uncropped images of blots are shown in Supplementary Fig. S4.
Figure 2
WWP2 regulates PTEN protein stability by polyubiquitylation. (a) Myc-tagged wild-type or a catalytically inactive C838A mutant of WWP2 were expressed in HeLa cells along with Flag–PTEN and HA–ubiquitin (Ub). 24 h post-transfection, cells were treated with MG132 (10 μM) for 6 h and the levels of PTEN ubiquitylation were evaluated by immunoprecipitation of PTEN using anti-Flag antibody followed by anti-HA immunoblotting. (b) A triple-tagged wild-type PTEN and the PTEN tyrosine mutants along with Myc–WWP2 were expressed in 293T cells and the level of PTEN–WWP2 interaction was detected by immunoprecipitation and immunoblotting with the indicated antibodies. The level of PTEN ubiquitylation was determined by immunoblotting with anti-ubiquitin antibodies. (c) HeLa cells were transfected with control siRNA or siRNAs against WWP2, EDD1 and NEDD4-1. Cell lysates prepared after 6 h MG132 (10 μM) treatment were subjected to immunoprecipitaton using anti-PTEN antibodies. The ubiquitylated PTEN was detected with anti-ubiquitin antibody. The protein expression and the specificity of different siRNAs were confirmed by immunoblotting of cell extracts using antibodies as indicated. (d) HeLa cells were transfected with control siRNA or siRNAs against WWP2, EDD1 and NEDD4-1. The protein levels of PTEN were assessed by immunoblotting using anti-PTEN antibody. (e) HeLa cells transiently expressing Flag-tagged PTEN were either transfected with plasmids encoding Myc-tagged WWP2 wild type or C838A mutant. Twenty-four hours post-transfection, cells were treated with cyclohexamide (CHX) and collected at the indicated times afterwards. The protein levels of PTEN were determined by anti-Flag immunoblotting. Uncropped images of blots are shown in Supplementary Fig. S4.
Figure 3
WWP2 activates AKT signalling and regulates stress-induced cell death in a PTEN-dependent manner. (a) DU145 prostate cancer cells were transfected with either control siRNA, WWP2 siRNA or a combination of WWP2 siRNA and PTEN siRNA. 72 h after siRNA transfection, cells were lysed and cell lysates were blotted with the indicated antibodies. Activation of AKT was detected by western blotting with antibody specific to AKT phosphorylated at Ser 473 (anti-pAKT). (b) DU145 prostate cancer cells transfected with the indicated siRNA were either left untreated or treated with doxorubicin. The percentage of apoptotic cells was measured after 36 h of treatment by using Annexin-V staining. The data shown are derived from three independent experiments (±s.d., for doxorubicin treatment P < 0.01 for cells expressing WWP2 siRNA, compared with cells expressing control siRNA or WWP2 and PTEN siRNA; Student’s _t_-test). (c) BPH1 (prostate epithelial-derived cell line) cells were transfected with either wild-type WWP2 or the C838A mutant. The cells were lysed and the activation of AKT was detected by western blotting with antibody specific to AKT phosphorylated at Ser 473 (anti-pAKT). Total AKT and expressed WWP2 proteins were detected by using AKT and Flag antibodies respectively. Actin was used as a loading control. (d) BPH1 cells transiently expressing wild-type or mutant WWP2 were either left untreated or treated with doxorubicin. After 36 h of treatment, apoptotic cells were determined by Annexin-V staining. The data shown are derived from three independent experiments (±s.d., for doxorubicin treatment P < 0.05 for cells expressing WWP2 wild-type, compared with mock treatment; Student’s _t_-test). Uncropped images of blots are shown in Supplementary Fig. S4.
Figure 4
WWP2 is required for tumorigenicity of cells. (a) DU145 cells were stably transfected with either retroviral-based control shRNA or two different WWP2 shRNAs. The expression levels of various proteins were analysed by immunoblotting with their respective antibodies. Actin was used as a loading control. (b) DU145 clones stably expressing control shRNA or WWP2 shRNAs were seeded and analysed for proliferation. The data shown are derived from four independent experiments (±s.d., P < 0.01, compared with cells expressing control shRNA; Student’s _t_-test). (c) DU145 cell lines stably expressing control shRNA or WWP2 shRNA were tested for anchorage-independent growth in a soft-agar colony assay. Viable colonies after 3 weeks were counted and the data (±s.d.) from three independent experiments were presented (P < 0.01, compared with cells expressing control shRNA; Student’s _t_-test). (d) Puromycin-resistant BPH1 prostate epithelial cells stably expressing either WWP2 wild type or the C838A mutant were established and the expression levels of PTEN and WWP2 were detected by the indicated antibodies. (e) A BPH1 parental cell line and BPH1-WWP2 wild-type or C838A mutant cells were analysed for proliferation in a similar way to that described in b. The data shown are derived from four independent experiments (±s.d., P < 0.05, compared with BPH1 parental cell line; Student’s _t_-test). (f) A non-transformed BPH1 cell line along with WWP2 wild-type- or C838A-mutant-expressing BPH1 cells were tested for anchorage-independent growth in a similar way to that described in c, and the data (±s.d.) were presented as summary of three independent experiments (P < 0.05, compared with BPH1 parental cell line; Student’s _t_-test). (g) DU145 stable clones expressing control shRNA or WWP2 shRNA alone or in combination with PTEN siRNA were analysed for proliferation in a similar way to that described in b. The data shown are derived from four independent experiments (±s.d., P < 0.01, compared with cells expressing control shRNA; Student’s _t_-test). (h) DU145 stable cell lines expressing control shRNA or WWP2 shRNA alone or in combination with PTEN siRNA were tested for anchorage-independent growth in a similar way to that described in c and the data (±s.d.) were presented as a summary of three independent experiments (P < 0.05, compared with cells expressing control shRNA; Student’s _t_-test). (i) Control shRNA- or WWP2 shRNA-expressing DU145 stable cells (5×106) were subcutaneously injected into nude mice and the tumour volumes were measured three times per week (±s.d., n = 5, P < 0.05, compared with cells expressing control shRNA; Student’s _t_-test). Uncropped images of blots are shown in Supplementary Fig. S4.
Similar articles
- Enzymatic Analysis of PTEN Ubiquitylation by WWP2 and NEDD4-1 E3 Ligases.
Chen Z, Thomas SN, Bolduc DM, Jiang X, Zhang X, Wolberger C, Cole PA. Chen Z, et al. Biochemistry. 2016 Jul 5;55(26):3658-66. doi: 10.1021/acs.biochem.6b00448. Epub 2016 Jun 22. Biochemistry. 2016. PMID: 27295432 Free PMC article. - WWP2 is a physiological ubiquitin ligase for phosphatase and tensin homolog (PTEN) in mice.
Li H, Zhang P, Zhang Q, Li C, Zou W, Chang Z, Cui CP, Zhang L. Li H, et al. J Biol Chem. 2018 Jun 8;293(23):8886-8899. doi: 10.1074/jbc.RA117.001060. Epub 2018 Apr 23. J Biol Chem. 2018. PMID: 29685889 Free PMC article. - The tumour suppressor PTEN mediates a negative regulation of the E3 ubiquitin-protein ligase Nedd4.
Ahn Y, Hwang CY, Lee SR, Kwon KS, Lee C. Ahn Y, et al. Biochem J. 2008 Jun 1;412(2):331-8. doi: 10.1042/BJ20071403. Biochem J. 2008. PMID: 18307411 - NEDD4: The founding member of a family of ubiquitin-protein ligases.
Boase NA, Kumar S. Boase NA, et al. Gene. 2015 Feb 25;557(2):113-22. doi: 10.1016/j.gene.2014.12.020. Epub 2014 Dec 17. Gene. 2015. PMID: 25527121 Free PMC article. Review. - The HECT family of E3 ubiquitin ligases and PTEN.
Song MS, Pandolfi PP. Song MS, et al. Semin Cancer Biol. 2022 Oct;85:43-51. doi: 10.1016/j.semcancer.2021.06.012. Epub 2021 Jun 12. Semin Cancer Biol. 2022. PMID: 34129913 Free PMC article. Review.
Cited by
- Poly-ADP ribosylation of PTEN by tankyrases promotes PTEN degradation and tumor growth.
Li N, Zhang Y, Han X, Liang K, Wang J, Feng L, Wang W, Songyang Z, Lin C, Yang L, Yu Y, Chen J. Li N, et al. Genes Dev. 2015 Jan 15;29(2):157-70. doi: 10.1101/gad.251785.114. Epub 2014 Dec 29. Genes Dev. 2015. PMID: 25547115 Free PMC article. - Recent advances in PTEN signalling axes in cancer.
Chow JT, Salmena L. Chow JT, et al. Fac Rev. 2020 Dec 23;9:31. doi: 10.12703/r/9-31. eCollection 2020. Fac Rev. 2020. PMID: 33659963 Free PMC article. Review. - E6AP/UBE3A ubiquitin ligase harbors two E2~ubiquitin binding sites.
Ronchi VP, Klein JM, Haas AL. Ronchi VP, et al. J Biol Chem. 2013 Apr 12;288(15):10349-60. doi: 10.1074/jbc.M113.458059. Epub 2013 Feb 25. J Biol Chem. 2013. PMID: 23439649 Free PMC article. - Ring finger protein 149 is an E3 ubiquitin ligase active on wild-type v-Raf murine sarcoma viral oncogene homolog B1 (BRAF).
Hong SW, Jin DH, Shin JS, Moon JH, Na YS, Jung KA, Kim SM, Kim JC, Kim KP, Hong YS, Lee JL, Choi EK, Lee JS, Kim TW. Hong SW, et al. J Biol Chem. 2012 Jul 6;287(28):24017-25. doi: 10.1074/jbc.M111.319822. Epub 2012 May 24. J Biol Chem. 2012. PMID: 22628551 Free PMC article. - Mechanism of PRL2 phosphatase-mediated PTEN degradation and tumorigenesis.
Li Q, Bai Y, Lyle LT, Yu G, Amarasinghe O, Nguele Meke F, Carlock C, Zhang ZY. Li Q, et al. Proc Natl Acad Sci U S A. 2020 Aug 25;117(34):20538-20548. doi: 10.1073/pnas.2002964117. Epub 2020 Aug 11. Proc Natl Acad Sci U S A. 2020. PMID: 32788364 Free PMC article.
References
- Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Current Cancer Drug Targets. 2008;8:187–198. - PubMed
- Li J, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. - PubMed
- Steck PA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 1997;15:356–362. - PubMed
- Guldberg P, et al. Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res. 1997;57:3660–3663. - PubMed
- Rhei E, et al. Mutation analysis of the putative tumour suppressor gene PTEN/MMAC1 in primary breast carcinomas. Cancer Res. 1997;57:3657–3659. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous