IL-12Rβ1 deficiency in two of fifty children with severe tuberculosis from Iran, Morocco, and Turkey - PubMed (original) (raw)
. 2011 Apr 13;6(4):e18524.
doi: 10.1371/journal.pone.0018524.
Jamila El Baghdadi, Nima Parvaneh, Aziz Bousfiha, Jacinta Bustamante, Jacqueline Feinberg, Arina Samarina, Audrey V Grant, Lucile Janniere, Naima El Hafidi, Amal Hassani, Daniel Nolan, Jilali Najib, Yildiz Camcioglu, Nevin Hatipoglu, Cigdem Aydogmus, Gonul Tanir, Caner Aytekin, Melike Keser, Ayper Somer, Guside Aksu, Necil Kutukculer, Davood Mansouri, Alireza Mahdaviani, Setareh Mamishi, Alexandre Alcais, Laurent Abel, Jean-Laurent Casanova
Affiliations
- PMID: 21533230
- PMCID: PMC3076373
- DOI: 10.1371/journal.pone.0018524
IL-12Rβ1 deficiency in two of fifty children with severe tuberculosis from Iran, Morocco, and Turkey
Stéphanie Boisson-Dupuis et al. PLoS One. 2011.
Abstract
Background and objectives: In the last decade, autosomal recessive IL-12Rβ1 deficiency has been diagnosed in four children with severe tuberculosis from three unrelated families from Morocco, Spain, and Turkey, providing proof-of-principle that tuberculosis in otherwise healthy children may result from single-gene inborn errors of immunity. We aimed to estimate the fraction of children developing severe tuberculosis due to IL-12Rβ1 deficiency in areas endemic for tuberculosis and where parental consanguinity is common.
Methods and principal findings: We searched for IL12RB1 mutations in a series of 50 children from Iran, Morocco, and Turkey. All children had established severe pulmonary and/or disseminated tuberculosis requiring hospitalization and were otherwise normally resistant to weakly virulent BCG vaccines and environmental mycobacteria. In one child from Iran and another from Morocco, homozygosity for loss-of-function IL12RB1 alleles was documented, resulting in complete IL-12Rβ1 deficiency. Despite the small sample studied, our findings suggest that IL-12Rβ1 deficiency is not a very rare cause of pediatric tuberculosis in these countries, where it should be considered in selected children with severe disease.
Significance: This finding may have important medical implications, as recombinant IFN-γ is an effective treatment for mycobacterial infections in IL-12Rβ1-deficient patients. It also provides additional support for the view that severe tuberculosis in childhood may result from a collection of single-gene inborn errors of immunity.
Conflict of interest statement
Competing Interests: The authors received funding from Talecris Biotherapeutics. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Figures
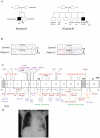
Figure 1. Mendelian mutations in IL12RB1 leading to severe tuberculosis in two kindreds.
A. Pedigree of the two families (A and B) with IL-12Rβ1 deficiency. Each generation is designated by a roman numeral (I–II), and each individual by an Arabic numeral. The double lines connecting the parents indicate consanguinity. The probands are indicated by an arrow, with black indicating Mycobacterium tuberculosis disease status. Individuals whose genetic status could not be evaluated are indicated by the symbol “E?”. B. Electrophoregram showing the genomic sequences of exons 9 and 5 in patients 1 and 2, respectively, compared with a control sequence. C. Schematic diagram of the coding region of the IL-12Rβ1 chain containing 17 coding exons and encoding a 662-amino acid protein with a leader sequence (exon1, L), extracellular domain (exons 2 to 13, EC), transmembrane domain (exon 14, TM) and an intracellular cytoplasmic domain (exons 15 to 17, IC). Published and unpublished mutations are indicated as follows: missense mutations are shown in purple, nonsense mutations are shown in red and complex mutations are shown in brown. Splicing mutations are shown in blue, large deletions are shown in green, insertions are shown in orange, and duplication is shown in magenta. * The 700+362_1619-944del mutation is the only mutation resulting in at the expression of a protein at the cell surface. Mutations of P1 (K305X) and P2 (R173W) are underlined. D. Chest X ray of patient 1 showing the localization of the disease.
Similar articles
- Susceptibility to mycobacterial disease due to mutations in IL-12Rβ1 in three Iranian patients.
Alinejad Dizaj M, Mortaz E, Mahdaviani SA, Mansouri D, Mehrian P, Verhard EM, Varahram M, Babaie D, Adcock IM, Garssen J, van de Vosse E, Velayati A. Alinejad Dizaj M, et al. Immunogenetics. 2018 Jun;70(6):373-379. doi: 10.1007/s00251-017-1041-3. Epub 2017 Dec 18. Immunogenetics. 2018. PMID: 29256176 Free PMC article. - Impaired IL-12- and IL-23-Mediated Immunity Due to IL-12Rβ1 Deficiency in Iranian Patients with Mendelian Susceptibility to Mycobacterial Disease.
Nekooie-Marnany N, Deswarte C, Ostadi V, Bagherpour B, Taleby E, Ganjalikhani-Hakemi M, Le Voyer T, Rahimi H, Rosain J, Pourmoghadas Z, Sheikhbahaei S, Khoshnevisan R, Petersheim D, Kotlarz D, Klein C, Boisson-Dupuis S, Casanova JL, Bustamante J, Sherkat R. Nekooie-Marnany N, et al. J Clin Immunol. 2018 Oct;38(7):787-793. doi: 10.1007/s10875-018-0548-1. Epub 2018 Sep 25. J Clin Immunol. 2018. PMID: 30255293 Free PMC article. - A Variety of Alu-Mediated Copy Number Variations Can Underlie IL-12Rβ1 Deficiency.
Rosain J, Oleaga-Quintas C, Deswarte C, Verdin H, Marot S, Syridou G, Mansouri M, Mahdaviani SA, Venegas-Montoya E, Tsolia M, Mesdaghi M, Chernyshova L, Stepanovskiy Y, Parvaneh N, Mansouri D, Pedraza-Sánchez S, Bondarenko A, Espinosa-Padilla SE, Yamazaki-Nakashimada MA, Nieto-Patlán A, Kerner G, Lambert N, Jacques C, Corvilain E, Migaud M, Grandin V, Herrera MT, Jabot-Hanin F, Boisson-Dupuis S, Picard C, Nitschke P, Puel A, Tores F, Abel L, Blancas-Galicia L, De Baere E, Bole-Feysot C, Casanova JL, Bustamante J. Rosain J, et al. J Clin Immunol. 2018 Jul;38(5):617-627. doi: 10.1007/s10875-018-0527-6. Epub 2018 Jul 11. J Clin Immunol. 2018. PMID: 29995221 Free PMC article. Review. - Molecular, Immunological, and Clinical Features of 16 Iranian Patients with Mendelian Susceptibility to Mycobacterial Disease.
Sarrafzadeh SA, Nourizadeh M, Mahloojirad M, Fazlollahi MR, Shokouhi Shoormasti R, Badalzadeh M, Deswarte C, Casanova JL, Pourpak Z, Bustamante J, Moin M. Sarrafzadeh SA, et al. J Clin Immunol. 2019 Apr;39(3):287-297. doi: 10.1007/s10875-019-0593-4. Epub 2019 Feb 4. J Clin Immunol. 2019. PMID: 30715640 - IL-12Rβ1 deficiency: mutation update and description of the IL12RB1 variation database.
van de Vosse E, Haverkamp MH, Ramirez-Alejo N, Martinez-Gallo M, Blancas-Galicia L, Metin A, Garty BZ, Sun-Tan Ç, Broides A, de Paus RA, Keskin Ö, Çağdaş D, Tezcan I, Lopez-Ruzafa E, Aróstegui JI, Levy J, Espinosa-Rosales FJ, Sanal Ö, Santos-Argumedo L, Casanova JL, Boisson-Dupuis S, van Dissel JT, Bustamante J. van de Vosse E, et al. Hum Mutat. 2013 Oct;34(10):1329-39. doi: 10.1002/humu.22380. Epub 2013 Aug 8. Hum Mutat. 2013. PMID: 23864330 Free PMC article. Review.
Cited by
- Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes.
Boisson-Dupuis S, Kong XF, Okada S, Cypowyj S, Puel A, Abel L, Casanova JL. Boisson-Dupuis S, et al. Curr Opin Immunol. 2012 Aug;24(4):364-78. doi: 10.1016/j.coi.2012.04.011. Epub 2012 May 30. Curr Opin Immunol. 2012. PMID: 22651901 Free PMC article. Review. - Partial IFN-γR2 deficiency is due to protein misfolding and can be rescued by inhibitors of glycosylation.
Moncada-Vélez M, Martinez-Barricarte R, Bogunovic D, Kong XF, Blancas-Galicia L, Tirpan C, Aksu G, Vincent QB, Boisson B, Itan Y, Ramírez-Alejo N, Okada S, Kreins AY, Bryant VL, Franco JL, Migaud M, Espinosa-Padilla S, Yamazaki-Nakashimada M, Espinosa-Rosales F, Kutukculer N, Abel L, Bustamante J, Vogt G, Casanova JL, Boisson-Dupuis S. Moncada-Vélez M, et al. Blood. 2013 Oct 3;122(14):2390-401. doi: 10.1182/blood-2013-01-480814. Epub 2013 Aug 20. Blood. 2013. PMID: 23963039 Free PMC article. - Human genetics of infectious diseases: Unique insights into immunological redundancy.
Casanova JL, Abel L. Casanova JL, et al. Semin Immunol. 2018 Apr;36:1-12. doi: 10.1016/j.smim.2017.12.008. Epub 2017 Dec 16. Semin Immunol. 2018. PMID: 29254755 Free PMC article. Review. - Lethal Infectious Diseases as Inborn Errors of Immunity: Toward a Synthesis of the Germ and Genetic Theories.
Casanova JL, Abel L. Casanova JL, et al. Annu Rev Pathol. 2021 Jan 24;16:23-50. doi: 10.1146/annurev-pathol-031920-101429. Epub 2020 Apr 14. Annu Rev Pathol. 2021. PMID: 32289233 Free PMC article. Review. - Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-γ immunity.
Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Bustamante J, et al. Semin Immunol. 2014 Dec;26(6):454-70. doi: 10.1016/j.smim.2014.09.008. Epub 2014 Oct 26. Semin Immunol. 2014. PMID: 25453225 Free PMC article. Review.
References
- Smith T. Vol. 196. Princeton University Press; 1934. Parasitism and disease,
- WHO. Vol. 2006. Geneva: WHO/HTM/TB/; 2006. Global tuberculosis control. Surveillance, planning, financing.362
- Ranke K. Diagnose und Epidemiologie der Lungentuberculose des Kindes. Archiv fuer Kinderheilkunde. 1910;54:279–306.
- von Pirquet C. Frequency of tuberculosis in childhood. JAMA. 1909;LII:675–8.
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI088685/AI/NIAID NIH HHS/United States
- UL1 RR024143/RR/NCRR NIH HHS/United States
- 5UL1RR024143/RR/NCRR NIH HHS/United States
- 1U01AI088685/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical