The A391E mutation enhances FGFR3 activation in the absence of ligand - PubMed (original) (raw)
The A391E mutation enhances FGFR3 activation in the absence of ligand
Fenghao Chen et al. Biochim Biophys Acta. 2011 Aug.
Abstract
The A391E mutation in the transmembrane domain of fibroblast growth factor receptor 3 leads to aberrant development of the cranium. It has been hypothesized that the mutant glutamic acid stabilizes the dimeric receptor due to hydrogen bonding and enhances its ligand-independent activation. We previously tested this hypothesis in lipid bilayers and showed that the mutation stabilizes the isolated transmembrane domain dimer by -1.3°kcal/mol. Here we further test the hypothesis, by investigating the effect of the A391E mutation on the activation of full-length fibroblast growth factor receptor 3 in human embryonic kidney 293T cells in the absence of ligand. We find that the mutation enhances the ligand-independent activation propensity of the receptor by -1.7°kcal/mol. This value is consistent with the observed strength of hydrogen bonds in membranes, and supports the above hypothesis.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
Fig 1
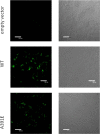
Immmunostaining of HEK 293T cells expressing wild-type and mutant FGFR3 at the plasma membrane. Cells were cultured for 24 hours after transfection and serum starved. After fixing with 4% paraformaldehyde (PFA), the cells were blocked using bovine serum albumin (BSA) for 1 hour. The cells were incubated with anti-FGFR3 antibodies, followed by FITC-conjugated goat anti rabbit IgG antibodies without cell permeabilization to identify wild-type and mutant receptors localized at the cell surface. (A) 10× objective; (B) 60× objective.
Fig 1
Immmunostaining of HEK 293T cells expressing wild-type and mutant FGFR3 at the plasma membrane. Cells were cultured for 24 hours after transfection and serum starved. After fixing with 4% paraformaldehyde (PFA), the cells were blocked using bovine serum albumin (BSA) for 1 hour. The cells were incubated with anti-FGFR3 antibodies, followed by FITC-conjugated goat anti rabbit IgG antibodies without cell permeabilization to identify wild-type and mutant receptors localized at the cell surface. (A) 10× objective; (B) 60× objective.
Fig. 2
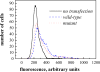
Flow cytometry data for HEK 293 T cells expressing wild-type and mutant FGFR3. Non-transfected cells served as control. FGFR3 expressed on the cell surface was probed using anti-N-FGFR3 antibodies, followed by fluorescein conjugated anti-rabbit IgG antibodies.
Fig 3
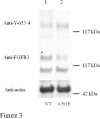
Expression and phosphorylation of wild-type FGFR3 and the A391E mutant in HEK 293T cells. HEK 293T cells were transfected with 1μg DNA encoding FGFR3/wt (lane 1 and FGFR3/A391E (lane 2). Cells were lysed after 24-hour culture and 24-hour starvation, and subjected to Western blotting. The blots were probed using anti-FGFR3 antibodies to assess total receptor levels (middle panel) and using anti-Y653/654 antibodies to assess receptor phosphorylation levels (top panel). Actin is shown in the bottom panel. Lanes have been removed for clarity.
Fig 4
Effect of fgf1 concentration on FGFR3 phosphorylation. (A) Representative Western blots, probed using anti-FGFR3 antibodies to assess total receptor levels (bottom) and using anti-Y653/654 antibodies to assess receptor phosphorylation (top). Top: cells transfected with 1 μg of the FGFR3/wt plasmid. Bottom: cells transfected with 2 μg of the FGFR/A391E plasmid. Cells were serum starved for 24 hours, then fgf1 was added for 10 minutes to the serum-free medium, at concentrations ranging from 5 ng/ml to 5000 ng/ml. The phosphorylation of the mature 130 kDa FGFR3 increased as the ligand concentration increased, and a plateau was reached above the concentration of 1000ng/ml. (B) Graphical analysis of ligand induced wild-type and A391E receptor phosphorylation. Receptor phosphorylation was proportional to the anti-Y653/654 band intensity and was assigned a value of 1 at saturating fgf1 concentrations.
Fig 4
Effect of fgf1 concentration on FGFR3 phosphorylation. (A) Representative Western blots, probed using anti-FGFR3 antibodies to assess total receptor levels (bottom) and using anti-Y653/654 antibodies to assess receptor phosphorylation (top). Top: cells transfected with 1 μg of the FGFR3/wt plasmid. Bottom: cells transfected with 2 μg of the FGFR/A391E plasmid. Cells were serum starved for 24 hours, then fgf1 was added for 10 minutes to the serum-free medium, at concentrations ranging from 5 ng/ml to 5000 ng/ml. The phosphorylation of the mature 130 kDa FGFR3 increased as the ligand concentration increased, and a plateau was reached above the concentration of 1000ng/ml. (B) Graphical analysis of ligand induced wild-type and A391E receptor phosphorylation. Receptor phosphorylation was proportional to the anti-Y653/654 band intensity and was assigned a value of 1 at saturating fgf1 concentrations.
Fig 5
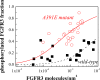
Active fraction of mature FGFR3 in the absence of ligand, as a function of its expression. Varying levels of receptor expression were achieved by varying the amount of plasmid used for transfection from 0.75μg to 6 μg of plasmid. Phosphorylated FGFR3 fractions were measured by comparing phosphorylation levels in the absence of ligand and at saturating fgf1 concentration (2500 ng/ml fgf1). The A391E mutant ( ) shows a higher phosphorylated fraction than the wild-type (□). The data were fitted to a simple activation model (see scheme (1) and reference (13)), yielding the activation propensities for the wild-type and mutant receptors. The effect of A391E on the activation propensity was calculated as −1.7 kcal/mol.
) shows a higher phosphorylated fraction than the wild-type (□). The data were fitted to a simple activation model (see scheme (1) and reference (13)), yielding the activation propensities for the wild-type and mutant receptors. The effect of A391E on the activation propensity was calculated as −1.7 kcal/mol.
Similar articles
- FGFR3 transmembrane domain interactions persist in the presence of its extracellular domain.
Sarabipour S, Hristova K. Sarabipour S, et al. Biophys J. 2013 Jul 2;105(1):165-71. doi: 10.1016/j.bpj.2013.05.053. Biophys J. 2013. PMID: 23823235 Free PMC article. - Effect of the achondroplasia mutation on FGFR3 dimerization and FGFR3 structural response to fgf1 and fgf2: A quantitative FRET study in osmotically derived plasma membrane vesicles.
Sarabipour S, Hristova K. Sarabipour S, et al. Biochim Biophys Acta. 2016 Jul;1858(7 Pt A):1436-42. doi: 10.1016/j.bbamem.2016.03.027. Epub 2016 Mar 31. Biochim Biophys Acta. 2016. PMID: 27040652 Free PMC article. - Multiple consequences of a single amino acid pathogenic RTK mutation: the A391E mutation in FGFR3.
Chen F, Sarabipour S, Hristova K. Chen F, et al. PLoS One. 2013;8(2):e56521. doi: 10.1371/journal.pone.0056521. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437153 Free PMC article. - [Cytokines in bone diseases. FGF receptor signaling and achondroplasia/hypochondroplasia].
Tanaka H. Tanaka H. Clin Calcium. 2010 Oct;20(10):1490-6. Clin Calcium. 2010. PMID: 20890030 Review. Japanese. - FGFR3 targeting strategies for achondroplasia.
Laederich MB, Horton WA. Laederich MB, et al. Expert Rev Mol Med. 2012 Jan 19;14:e11. doi: 10.1017/erm.2012.4. Expert Rev Mol Med. 2012. PMID: 22559284 Review.
Cited by
- Towards generalizable predictions for G protein-coupled receptor variant expression.
Kuntz CP, Woods H, McKee AG, Zelt NB, Mendenhall JL, Meiler J, Schlebach JP. Kuntz CP, et al. Biophys J. 2022 Jul 19;121(14):2712-2720. doi: 10.1016/j.bpj.2022.06.018. Epub 2022 Jun 17. Biophys J. 2022. PMID: 35715957 Free PMC article. - FGFR3 overactivation in the brain is responsible for memory impairments in Crouzon syndrome mouse model.
Cornille M, Moriceau S, Khonsari RH, Heuzé Y, Loisay L, Boitez V, Morice A, Arnaud E, Collet C, Bensidhoum M, Kaci N, Boddaert N, Paternoster G, Rauschendorfer T, Werner S, Mansour SL, Di Rocco F, Oury F, Legeai-Mallet L. Cornille M, et al. J Exp Med. 2022 Apr 4;219(4):e20201879. doi: 10.1084/jem.20201879. Epub 2022 Mar 7. J Exp Med. 2022. PMID: 35254402 Free PMC article. - Molecular investigation of FGFR3 gene mutation and its correlation with clinicopathological findings in Indian bladder cancer patients.
Ahmad F, Mahal V, Verma G, Bhatia S, Das BR. Ahmad F, et al. Cancer Rep (Hoboken). 2018 Oct;1(3):e1130. doi: 10.1002/cnr2.1130. Epub 2018 Sep 17. Cancer Rep (Hoboken). 2018. PMID: 32721083 Free PMC article. - ETV5 links the FGFR3 and Hippo signalling pathways in bladder cancer.
di Martino E, Alder O, Hurst CD, Knowles MA. di Martino E, et al. Sci Rep. 2019 Apr 5;9(1):5740. doi: 10.1038/s41598-018-36456-3. Sci Rep. 2019. PMID: 30952872 Free PMC article. - Pathogenic Cysteine Removal Mutations in FGFR Extracellular Domains Stabilize Receptor Dimers and Perturb the TM Dimer Structure.
Sarabipour S, Hristova K. Sarabipour S, et al. J Mol Biol. 2016 Oct 9;428(20):3903-3910. doi: 10.1016/j.jmb.2016.08.026. Epub 2016 Sep 3. J Mol Biol. 2016. PMID: 27596331 Free PMC article.
References
- Wilkie AOM, Morriss-Kay GM, Jones EY, Heath JK. Functions of fibroblast growth factors and their receptors. Curr. Biol. 1995;5:500–507. - PubMed
- L'Horte CGM, Knowles MA. Cell responses to FGFR3 signaling: growth, differentiation and apoptosis. Experim. Cell Res. 2005;304:417–431. - PubMed
- Vajo Z, Francomano CA, Wilkin DJ. The molecular and genetic basis of fibroblast growth factor receptor 3 disorders: The achondroplasia family of skeletal dysplasias, Muenke craniosynostosis, and Crouzon syndrome with acanthosis nigricans. Endocrine Reviews. 2000;21:23–39. - PubMed
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. - PubMed
- Harada D, Yamanaka Y, Ueda K, Tanaka H, Seino Y. FGFR3-related dwarfism and cell signaling. Journal of Bone and Mineral Metabolism. 2009;27:9–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical