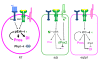Prospero and Pax2 combinatorially control neural cell fate decisions by modulating Ras- and Notch-dependent signaling - PubMed (original) (raw)
Figure 1
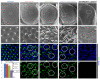
Pros and dPax2 together control cone cell development and lens formation. (A,B,D-K) Scanning electron micrographs (SEMs) of adult eyes (A,D,F,H,J) or immunostaining of 45% pupal retinas for the CC-specific marker Cut (green) and the membrane marker E-cadherin (blue) (B,E,G,I,K) were analyzed from the following genotypes: yw67; Sp/CyO; TM2/TM6B (A,B), eyflp3.5; Sp/CyO; FRT82_ubi_-GFPnls, RpS3/FRT82B-_pros_17 (D,E), yw67; Sp/CyO; TM2/TM6B; spapol/spapol(F,G), yw67; Sp/CyO; FRT82B-_pros_17/TM6B; spapol/spapol(H,I), and UAS-_d_Pax2RNAi; sev-GAL4/CyO; UAS-Pros RNAi/TM6B (J,K). A slightly more severe phenotype from that shown in (J,K) is apparent in _pros_17/_spapol_double mutants, but these eyes collapsed during SEM and were particularly difficult to dissect. Panels (A',D',F',H',J') represent the boxed areas in the corresponding larger SEM. Circles represent CC clusters within individual ommatidia, and the dotted circle in (E) represents a rare yet significant loss of one CC observed in pros mutants. Control eyes have regular lens facets by SEM (A) and four CCs per ommatida by Cut and E-cadherin double-staining (B). (C) Quantification of CC numbers shows a strong genetic interaction and dual requirement for Pros and dPax2 in CC specification. *P < 0.05, **_P_ < 0.001, n.d. = none detected. Error bars represent standard deviation. (D,E) _pros17_Minute LOF eyes (see Materials and methods) show mild roughening by SEM (D), and a rare yet significant loss of one CC (E, dotted circle). (F,G) _spapol_eyes show some roughening (F), consistent with a regular loss in PPCs [28] and at least one CC (G,G', circles). Removing one copy of _pros_ from _spapol_mutants causes further perturbation of lens morphogenesis, with holes in the center of the lenses frequently observed (H), and CC number is reduced to approximately two per ommatidia (I). _sev>_dPax2RNAi + ProsRRNAi lack lens facets by SEM (J) and Cut-positive cells are not detected (K).
Figure 2
Distinct subsets of cone cells are present in the eye imaginal disc. (A,C,E-G) In wild-type late third instar eye imaginal discs, Pros (A,E,F, magenta), dPax2 (C,F, blue), and Cut (A,C,E, green; G, white) were analyzed by immunostaining. Pros is higher in the equatorial (Eq) and polar (Pl) CCs, while the expression of Cut (E,G, green) and dPax2 (G, blue) are elevated in the anterior (A) and posterior (P) CCs. (B,D) These expression patterns represented in diagrams. Individual ommatidia are circled; anterior is left. (E) In _spapol_eye imaginal discs, Cut is mostly absent until the last few most posterior rows, while Pros expression (E, magenta; E', white) initiates in all CC precursors (circles). (F) In _pros17_mitotic clones, dPax2 expression (F, blue; F', white) is unchanged between pros mutant (Pros-negative, -/-) and wild-type (Pros-positive, magenta, +/+) tissue. (G) Cut expression is specifically delayed in Eq/Pl CCs (dotted circles) in _pros17_mutant tissue (-/-) (individual ommatidial CC clusters are highlighted with dashed circles), whereas in control tissue (+/+), a normal complement of four CCs is observed in comparably positioned ommatidia (solid circles).
Figure 3
Pros and dPax2 are sufficient to recruit cone cells, but from different cell populations. (A,B,E,F,I,J,M) Retinas from 45% pupa (A,B,E,F,I,J,M) were immunostained for CC nuclei (Cut, green in (A,A',E,E',I,I',M,M')), PR nuclei (Otd, magenta in (A,A'E,E'I,I',M,M')), and PPC nuclei (BarH1, green in (B,F,J) or white in (B',F',J')), and cell outlines were revealed by Dlg staining (black in (B,F,J)). (C,D,G,H,K,L) Adult eyes were stained with toluidine blue (C,G,K) or immunostained (D,H,L) with the R7 opsins Rh3 (magenta) and Rh4 (blue) and fluorescently labeled phalloidin was used to mark the actin-rich apical surfaces of photoreceptors (green). (N) Quantification of PRs (magenta), CCs (green), and PPCs (blue). (O) Summary of phenotypes observed with the gain-of-function (GOF) experiments (top) and wild-type Pros and dPax2 expression patterns (bottom). MC, mystery cells [35], a potential source of ectopic CCs in the double GOF experiments. (A-D) Control pupal eyes form eight PRs (A,A'), four CCs (A,A'), and two PPCs (B,B'), and adult eyes form a trapezoid of six large outer PR rhabdomeres surrounding a smaller, central R7 PR (C) expressing Rh3 and Rh4 (D). (E,F) In _sev>_Pros pupal retinas, ectopic Cut-positive CCs are observed in ommatidia with a full complement of PRs (E, circle), and only one PPC is commonly observed (F,F', circle). Ommatidia with a complement of four CCs occasionally form an extra R7 (E, dotted circle; G,H). Ectopic CCs are also observed in _sev_>dPax2 pupal retinas (I,I'). PPCs are disorganized in _sev_>dPax2 retinas, but do not change significantly in number (J,J',N). PR number is reduced by one in _sev_>dPax2 pupal retinas (I,I') and R7s are frequently absent from adult eyes (K,L). (M) In _sev_>pros+dPax2 pupal eyes, one ectopic CC per ommatidia is frequently observed (circle), but PR and PPC numbers remain unchanged (N) (also see Additional file 1C,D). *P < 0.05, **P < 0.001. Error bars represent standard deviation.
Figure 4
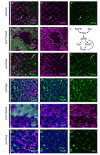
Pros is necessary and sufficient for high pERK levels. (A,B,D-G) Eye imaginal discs from control (A,E), _pros1_7mitotic clones (B,F) and _sev_>Pros eyes (D,G) were immunostained for Pros (A,B, green; E,G, magenta), pERK (A,B,D, magenta), and Yan (E,G, green; F, magenta). GFP was used to mark wild-type (versus _pros1_7mutant) tissue (B,F, green), and nuclei were visualized with DAPI (E,F,G, blue). (C) A diagram representing Pros regulation by Ras/MAPK signaling previously reported [26,27,29], and the positive feedback onto pERK described here. In optical sections at the level of the R7 and CCs, high Pros expression correlates with high pERK expression (A,C, arrows), whereas a cell autonomous reduction in pERK is observed in pros mutant tissue (B, non-GFP positive cells). No change in pERK is observed in more basal optical sections where Pros is not expressed (data not shown). (E) In wild-type imaginal discs, Yan expression (green) becomes reduced as Pros expression (magenta) increases in specified CCs. (F) In pros mutants, Yan (magenta) is only present in nuclei in GFP-negative (that is, _pros_-negative) tissue, whereas it is both nuclear and cytoplasmic in surrounding GFP-positive wild-type tissue (green). (G) In _sev_>Pros imaginal discs, Yan (green) is reduced throughout the disc, being almost undetectable in cells expressing particularly high levels of Pros (magenta).
Figure 5
dPax2 effects Delta/Notch signaling. (A-F) Eye imaginal discs from control (A,C), _spapol_mutants (B,C), _mirr_>GFP (E), and _mirr_>GFP,dPax2RNAi were immunostained for Delta (A,B, green), E-cadherin (A,B, blue), LacZ (C,D, green), Pros (C,D, magenta), GFP (E,F, green), or E(spl) (E,F, magenta). All discs are oriented with anterior left. In wild-type discs, Delta expression is high in the first four rows of ommatidia after the morphogenetic furrow (MF) and then decreases thereafter (A). In _spapol_mutants, however, Delta is up-regulated again by row 7. Delta-LacZ expression in wild-type discs is down-regulated at the more posterior rows of the disc and do not co-localize with Pros in CC precursors (C), whereas in spapol mutants, Delta-LacZ expression is maintained at high levels throughout the disc and co-localizes with Pros in the most posterior rows (D), indicating that dPax2 transcriptionally represses Delta expression in CC precursors. E(spl) (E,F) is equally expressed when a UAS-GFP transgene is misexpressed in the dorsal half of the eye imaginal disc with _mirr_-GAL4 (E), but is significantly reduced where UAS-dPax2RNAi/UAS-GFP are co-expressed (F), revealing that dPax2 is important for maintaining high Notch activity.
Figure 6
dPax and Pros control the neuronal to non-neuronal switch in the R7 equivalence group. (A-D) In eyes overexpressing Pros in the absence of dPax2 (_sev>_Pros; _spapol_eyes), lenses are almost absent by SEM (A,B), no Cut-positive CCs are observed (C, green), and R7 number is significantly increased, with three to four often present in individual ommatidia (D, circles). (E-H) In eyes overexpressing dPax2 in the absence of Pros (_sev>_dPax2+_prosRNAi_eyes), lens formation is mildly disrupted (E,F), an average of five CCs/ommatidia are formed (G, circles), and R7 PRs are rarely observed (H). (I) Quantification of PR (magenta) and CC (green) numbers in pros and dPax2 LOF and GOF experiments (see Table 1 for values, and Materials and methods for specific genotypes). Error bars represent standard deviation
Figure 7
Proposed model for the roles of Pros and dPax2 during cell fate decisions in the R7 equivalence group. Model depicting the outcome of Ras/MAPK and Notch (N/Delta (Dl signaling in the R7, anterior (a)/posterior (p) CCs, and equatorial (eq)/polar (pl) CCs based on the differences in Pros and dPax2 observed in these different cell populations. Phyllopod (Phyl) has been previously suggested to prevent dPax2 expression in the presumptive R7 [34]. In this model, the a/p CCs would be dominated by dPax2/Cut/Notch activity, whereas the eq/pl CCs would be dominated by Pros/pERK/Delta. Based on our LOF analysis, we propose that a/p and eq/pl CCs represent two distinct CCs that are inter-convertible. Moreover, in pros mutants, we postulate that four 'a/p' CCs form, whereas in dPax2 mutants, 'eq/pl' CCs form.