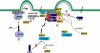Mechanism of inhibition of retrovirus release from cells by interferon-induced gene ISG15 - PubMed (original) (raw)
Mechanism of inhibition of retrovirus release from cells by interferon-induced gene ISG15
Zhizhou Kuang et al. J Virol. 2011 Jul.
Abstract
Budding of retroviruses from cell membranes requires ubiquitination of Gag and recruitment of cellular proteins involved in endosome sorting, including endosome sorting complex required for transport III (ESCRT-III) protein complex and vacuolar protein sorting 4 (VPS4) and its ATPase. In response to infection, a cellular mechanism has evolved that blocks virus replication early and late in the budding process through expression of interferon-stimulated gene 15 (ISG15), a dimer homologue of ubiquitin. Interferon treatment of DF-1 cells blocks avian sarcoma/leukosis virus release, demonstrating that this mechanism is functional under physiological conditions. The late block to release is caused in part by a loss in interaction between VPS4 and its coactivator protein LIP5, which is required to promote the formation of the ESCRT III-VPS4 double-hexamer complex to activate its ATPase. ISG15 is conjugated to two different LIP5-ESCRT-III-binding charged multivesicular body proteins, CHMP2A and CHMP5. Upon ISGylation of each, interaction with LIP5 is no longer detected. Two other ESCRT-III proteins, CHMP4B and CHMP6, are also conjugated to ISG15. ISGylation of CHMP2A, CHMP4B, and CHMP6 weakens their binding directly to VPS4, thereby facilitating the release of this protein from the membrane into the cytosol. The remaining budding complex fails to release particles from the cell membrane. Introducing a mutant of ISG15 into cells that cannot be conjugated to proteins prevents the ISG15-dependent mechanism from blocking virus release. CHMP5 is the primary switch to initiate the antiviral mechanism, because removal of CHMP5 from cells prevents ISGylation of CHMP2A and CHMP6.
Figures
Fig. 1.
Expression of ISG15 and its ligase complex induced by transfection or interferon treatment of DF-1 cells blocked virus release. (A) The RCAN vector was transfected into DF-1 cells, and the appearance of ASLV in the culture supernatants was determined by RT activity in detergent-activated virions as described in Materials and Methods. ⋄, RCAN-transfected cells; ◊, untransfected cells. (B) DF-1 cells or DF-1 cells at 25 days posttransfection with RCAN vectors were transfected with pHis-ISG15 or the pHis-ISG15 mutant form containing a GG-to-AA substitution plus the E1, E2, and E3 ligase plasmids or treated with rChIFN-α (2,000 units) as described in Materials and Methods. At 48 h after transfection or treatment with rChIFN-α, ASLV in the medium fraction was analyzed as in panel A. His-ISG15 and His-ISG15 GG-to-AA mutant expression in lysates was detected by Western blotting (WB) with anti-ISG15 serum (lower panel). Anti-actin serum was used to detect β-actin as a loading control. (C) DF-1 cells or DF-1 cells at 25 days posttransfection with RCAN vectors were transfected with pHis-ISG15 or treated with chicken interferon along with an siRNA pool (40 nM or 80 nM concentration) targeting CHMP5 (CH5) or a nontargeting siRNA (80 nM) control (CONT). Forty-eight hours after transfection or treatment with interferon, ASLVs in the medium fraction were collected and analyzed as in panel A. To confirm knockdown of CHMP5 in DF-1 cells, endogenous CHMP5 expression in cells in the presence of control siRNA (80 nM; lower panel, lane 1) or the siRNA targeting CHMP5 (40 nM, lane 2; 80 nM, lane 3) was analyzed by Western blotting with anti-ISG15 serum. β-Actin again served as a loading control. The RT activity data in panels A, B, and C represent the averages from three independent experiments.
Fig. 2.
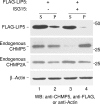
ISG15 expression blocks the interaction of LIP5 with CHMP5 and CHMP2A but not CHMP3. (A) 293E cells were transfected with plasmids expressing FLAG-LIP5 and/or HA-CHMP5 (lanes 1 and 2) and, where indicated, pHis-ISG15, E1, and E2 (lane 3) as described in Materials and Methods. Forty-eight hours posttransfection, 10% of total cell lysate input was resolved by 10% SDS-PAGE to verify expression of FLAG-LIP5 and HA-CHMP5 by Western blotting with anti-HA or anti-FLAG serum (bottom panels, as indicated). The lysate was probed with an anti-actin serum as a loading control. FLAG-LIP5 was immunoprecipitated (IP) from the remainder of the cell lysate fraction with an anti-FLAG serum and resolved by 10% SDS-PAGE. HA-CHMP5 was detected by Western blotting with an anti-HA serum (top panel). (B) 293E cells were transfected with plasmids expressing FLAG-LIP5 and ISG15, E1, and E2 where indicated. Cell lysates were prepared, endogenous CHMP5 and CHMP2A were immunoprecipitated with the respective antisera, and samples were analyzed as in panel A. (C) 293E cells were transfected with plasmids expressing FLAG-LIP5 and HA-CHMP2A (lane 1 to 3) or HA-CHMP3 (lanes 4 to 6). Expression plasmids for pHis-ISG15, E1, E2, and E3 were cotransfected into cells where indicated (lane 3 and lane 6). Samples were analyzed as in panel A except that HA-CHMP2A and HA-CHMP3 were immunoprecipitated with an antiserum directed at the respective proteins and an anti-HA or -FLAG serum was used in Western blotting to detect the three proteins (top panel). Molecular size markers (in kilodaltons) are indicated by the numbers at the sides of the panels.
Fig. 3.
ISG15 expression results in release of LIP5 from the budding complex in membranes into the cytosol. 293E cells were transfected with plasmids expressing FLAG-LIP5 and, where indicated, pHis-ISG15, E1, and E2. Forty-eight hours posttransfection, cell lysates were prepared and fractionated into soluble and pellet fractions as described in Materials and Methods. Distribution of FLAG-LIP5 and endogenous CHMP2A or CHMP5 in the resulting soluble (S) (lanes 1 and 3) and membrane pellet (P) (lanes 2 and 4) fractions were visualized by Western blotting with anti-FLAG serum to detect LIP5 or anti-CHMP2A and anti-CHMP5 sera to detect endogenous CHMP2A and CHMP5, respectively. β-Actin served as a loading control.
Fig. 4.
ISG15 conjugates to ESCRT-III proteins. (A) ISG15 conjugates to CHMP2A, CHMP4B, and CHMP6. 293E cells were transfected with plasmids expressing C-terminal FLAG-tagged CHMP2A, -3, -4B, -5, or -6 in the presence or absence of a plasmid expressing CHMP-ISG15, E1, and E2 ligases as described in Materials and Methods. Forty-eight hours posttransfection, cells were lysed and ISGylated proteins were affinity purified using Ni+-agarose beads as described in Materials and Methods. Proteins were resolved by 12% SDS-PAGE. ISGylated CHMP5 (lane 2), CHMP2A (lane 4), CHMP4B (lane 8), and CHMP6 (lane 10) were visualized by Western blot analysis with a monoclonal anti-FLAG serum (top panel). No CHMP3-ISG15 conjugate was detected (lane 6). The bottom panel shows a Western blot of the 10% input of total cell lysates used in the affinity purification step, to verify expression of CHMP5-FLAG (lanes 1 and 2), CHMP2A-FLAG (lanes 3 and 4), CHMP3-FLAG (lanes 5 and 6), CHMP4B-FLAG (lanes 7 and 8), and CHMP6-FLAG (lanes 9 and 10), respectively. β-Actin served as a loading control. (B) ISG15 also conjugates to CHMP3 and is blocked by the ISG15 GG-to-AA mutant. 293E cells were transfected with plasmids expressing HA-CHMP2A or -3 in the presence or absence of a plasmid expressing His-ISG15 or the His-ISG15 GG-to-AA mutant and E1, E2, and E3 as described in Materials and Methods. Samples were analyzed as in panel A except that HA-CHMP2A and HA-CHMP3 were detected by using an anti-HA serum. GG denotes His-tagged wild-type ISG15; AA denotes the His-tagged ISG15 mutant.
Fig. 5.
ISG15 expression weakens the interaction of VPS4 with ESCRT-III proteins. (A) 293E cells were cotransfected with plasmids expressing HA-VPS4 and FLAG-tagged CHMP1B, CHMP2A, or CHMP3 and 3 μg of pHis-ISG15 where indicated. At 48 h posttransfection, HA-VPS4 was immunoprecipitated from cell extracts with an anti-HA serum, and samples were resolved by 10% SDS-PAGE. For the top panel, precipitation of CHMP1B-FLAG (lanes 1 to 3), CHMP2A-FLAG (lanes 4 to 6), and CHMP3-FLAG (lanes 7 to 9) was determined by Western blotting with anti-FLAG antibody. The bottom panel shows a Western blot of the 10% input of total cell lysates used in the coimmunoprecipitation assay to verify even expression of HA-VPS4 (lanes 2 and 3, 5 and 6, and 8 and 9) and CHMP1B-FLAG (lanes 1 to 3), CHMP2A-FLAG (lanes 4 to 6), and CHMP3-FLAG (lanes 7 to 9), respectively. β-Actin served as a loading control. (B) 293E cells were transfected as described in the legend to panel A except that expression vectors of CHMP4B-FLAG (lanes 1 and 2) and CHMP6-FLAG (lanes 3 and 4) were substituted for the other ESCRT-III proteins. These experiments were repeated three different times with similar results.
Fig. 6.
Removal of CHMP5 from cells prevents ISGylation of CHMP2A and CHMP6. CHMP2A-FLAG or CHMP6-FLAG was coexpressed in 293E cells with ISG15, E1, and E2 as described in the legend to Fig. 1. Where indicated, an siRNA targeting CHMP5 was introduced into the cells as previously described (21). CHMP2A-FLAG and CHMP6-FLAG and their ISGylated forms were detected by Western blotting using an anti-FLAG serum (upper panel). Endogenous CHMP5 was detected by Western blotting using an anti-CHMP5 serum (middle panel). β-Actin was detected (lower panel) as a sample loading control as described in the legend to Fig. 2.
Fig. 7.
Mechanism of inhibition of late budding caused by ISG15 expression in cells. (Step 1) The ESCRT-III protein, CHMP5, binds to LIP5 in the cytosol. However, in the presence of ISG15-specific E1, E2, and E3 enzymes, ISG15 conjugates to CHMP5 and the binding to LIP5 is lost. (Step 2) The CHMP5-ISG15 conjugate accumulates in the budding complex on the membrane without LIP5. The interaction on the membrane between VPS4 and its coactivator LIP5 is thereby blocked, preventing activation of the ATPase through formation of the VPS4/LIP5 double-hexamer structure. (Step 3) CHMP2A is ISGylated in the presence of ISG15-CHMP5. This results in the loss of its binding to LIP5 and the weakening of its direct interaction to VPS4. (Step 4) CHMP6 is ISGylated in the presence of ISG15-CHMP5. This results in weakening of its binding to VPS4. (Step 5) VPS4 is released into the cytosol, and virus budding is arrested.
Similar articles
- The interferon-induced gene ISG15 blocks retrovirus release from cells late in the budding process.
Pincetic A, Kuang Z, Seo EJ, Leis J. Pincetic A, et al. J Virol. 2010 May;84(9):4725-36. doi: 10.1128/JVI.02478-09. Epub 2010 Feb 17. J Virol. 2010. PMID: 20164219 Free PMC article. - Novel interactions of ESCRT-III with LIP5 and VPS4 and their implications for ESCRT-III disassembly.
Shim S, Merrill SA, Hanson PI. Shim S, et al. Mol Biol Cell. 2008 Jun;19(6):2661-72. doi: 10.1091/mbc.e07-12-1263. Epub 2008 Apr 2. Mol Biol Cell. 2008. PMID: 18385515 Free PMC article. - A novel mechanism of regulating the ATPase VPS4 by its cofactor LIP5 and the endosomal sorting complex required for transport (ESCRT)-III protein CHMP5.
Vild CJ, Li Y, Guo EZ, Liu Y, Xu Z. Vild CJ, et al. J Biol Chem. 2015 Mar 13;290(11):7291-303. doi: 10.1074/jbc.M114.616730. Epub 2015 Jan 30. J Biol Chem. 2015. PMID: 25637630 Free PMC article. - The antiviral activities of ISG15.
Morales DJ, Lenschow DJ. Morales DJ, et al. J Mol Biol. 2013 Dec 13;425(24):4995-5008. doi: 10.1016/j.jmb.2013.09.041. Epub 2013 Oct 3. J Mol Biol. 2013. PMID: 24095857 Free PMC article. Review. - Interferon-stimulated gene 15 and the protein ISGylation system.
Zhang D, Zhang DE. Zhang D, et al. J Interferon Cytokine Res. 2011 Jan;31(1):119-30. doi: 10.1089/jir.2010.0110. Epub 2010 Dec 29. J Interferon Cytokine Res. 2011. PMID: 21190487 Free PMC article. Review.
Cited by
- HIV-1 assembly, budding, and maturation.
Sundquist WI, Kräusslich HG. Sundquist WI, et al. Cold Spring Harb Perspect Med. 2012 Jul;2(7):a006924. doi: 10.1101/cshperspect.a006924. Cold Spring Harb Perspect Med. 2012. PMID: 22762019 Free PMC article. Review. - Virus budding and the ESCRT pathway.
Votteler J, Sundquist WI. Votteler J, et al. Cell Host Microbe. 2013 Sep 11;14(3):232-41. doi: 10.1016/j.chom.2013.08.012. Cell Host Microbe. 2013. PMID: 24034610 Free PMC article. Review. - Antiviral Activity and Adaptive Evolution of Avian Tetherins.
Krchlíková V, Fábryová H, Hron T, Young JM, Koslová A, Hejnar J, Strebel K, Elleder D. Krchlíková V, et al. J Virol. 2020 Jun 1;94(12):e00416-20. doi: 10.1128/JVI.00416-20. Print 2020 Jun 1. J Virol. 2020. PMID: 32238588 Free PMC article. - ISG15 deficiency and increased viral resistance in humans but not mice.
Speer SD, Li Z, Buta S, Payelle-Brogard B, Qian L, Vigant F, Rubino E, Gardner TJ, Wedeking T, Hermann M, Duehr J, Sanal O, Tezcan I, Mansouri N, Tabarsi P, Mansouri D, Francois-Newton V, Daussy CF, Rodriguez MR, Lenschow DJ, Freiberg AN, Tortorella D, Piehler J, Lee B, García-Sastre A, Pellegrini S, Bogunovic D. Speer SD, et al. Nat Commun. 2016 May 19;7:11496. doi: 10.1038/ncomms11496. Nat Commun. 2016. PMID: 27193971 Free PMC article. - Viruses go modular.
Shepley-McTaggart A, Fan H, Sudol M, Harty RN. Shepley-McTaggart A, et al. J Biol Chem. 2020 Apr 3;295(14):4604-4616. doi: 10.1074/jbc.REV119.012414. Epub 2020 Feb 28. J Biol Chem. 2020. PMID: 32111739 Free PMC article. Review.
References
- Azmi I. F., et al. 2008. ESCRT-III family members stimulate VPS4 ATPase activity directly or via Vta1. Dev. Cell 14:50–61 - PubMed
- Babst M. 2005. A protein's final ESCRT. Traffic 6:2–9 - PubMed
- Dastur A., Beaudenon S., Kelley M., Klug R., Huibregtse J. 2006. Herc5, an interferon-induced HECT E3 enzyme, is required for conjugation of ISG15 in human cells. J. Biol. Chem. 281:4334–4338 - PubMed
- Demirov D. G., Freed E. O. 2004. Retrovirus budding. Virus Res. 106:87–102 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous