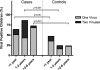Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values - PubMed (original) (raw)
Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values
Rogier R Jansen et al. J Clin Microbiol. 2011 Jul.
Abstract
Highly sensitive techniques, such as PCR, have greatly improved the detection of respiratory viruses. However, the sensitivity of PCR tests also complicates clinical interpretation, as the presence of small amounts of viral targets may not necessarily have clinical relevance. We performed a prospective case-control study in asymptomatic and symptomatic young children. PCR detection of 14 respiratory viruses was performed in nasal washes, and results were quantified in copies per milliliter. A total of 141 cases and 157 controls were included. In 72% of the cases and 28% of the controls, at least one virus was identified. When stratified for age, at least one virus was identified in 47% of the controls younger than 1 year old. Rhinovirus (RV) was frequently detected in both symptomatic and asymptomatic individuals. Receiver operating characteristic analysis for quantitative rhinovirus detection showed that cutoff values for clinical relevance are feasible for RV. In contrast to rhinovirus, respiratory syncytial virus (RSV) was rarely detected in controls, suggesting that a positive RSV test result is almost always of clinical relevance, independent of viral quantity. In conclusion, our study shows that asymptomatic carriage of a respiratory virus occurs frequently in young children. However, significant differences in the amount of virus present were observed between cases and controls. This suggests that defining cutoff levels should be feasible and represents the next necessary step for diagnosing viral respiratory infections using molecular tests.
Figures
Fig. 1.
Viral prevalence in cases and controls, stratified by age. The percentage represents the amount of positive cases for the total group and for each specific age group. Data for single infections and double infections (i.e., two or more virus species detected) are shown.
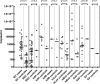
Fig. 2.
Crossing point values for each positive sample. White diamonds denote single infections, and black diamonds denote positive samples as part of a double or triple infection. Horizontal lines represent the median. PIV 1, PIV2, PIV3, PIV4, and hMPV are not shown in this figure because no positive samples were found among controls for these species.
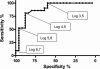
Fig. 3.
ROC curve for RV-positive samples, excluding double infections. The curve shows the relationship between sensitivity and specificity when different cutoffs in crossing point values for association with clinical illness were applied.
Similar articles
- Symptomatic and asymptomatic respiratory viral infections in the first year of life: association with acute otitis media development.
Chonmaitree T, Alvarez-Fernandez P, Jennings K, Trujillo R, Marom T, Loeffelholz MJ, Miller AL, McCormick DP, Patel JA, Pyles RB. Chonmaitree T, et al. Clin Infect Dis. 2015 Jan 1;60(1):1-9. doi: 10.1093/cid/ciu714. Epub 2014 Sep 9. Clin Infect Dis. 2015. PMID: 25205769 Free PMC article. - Rapid detection of respiratory tract viral infections and coinfections in patients with influenza-like illnesses by use of reverse transcription-PCR DNA microarray systems.
Renois F, Talmud D, Huguenin A, Moutte L, Strady C, Cousson J, Lévêque N, Andréoletti L. Renois F, et al. J Clin Microbiol. 2010 Nov;48(11):3836-42. doi: 10.1128/JCM.00733-10. Epub 2010 Aug 25. J Clin Microbiol. 2010. PMID: 20739481 Free PMC article. - Improved detection of respiratory viruses in pediatric outpatients with acute respiratory illness by real-time PCR using nasopharyngeal flocked swabs.
Munywoki PK, Hamid F, Mutunga M, Welch S, Cane P, Nokes DJ. Munywoki PK, et al. J Clin Microbiol. 2011 Sep;49(9):3365-7. doi: 10.1128/JCM.02231-10. Epub 2011 Jul 20. J Clin Microbiol. 2011. PMID: 21775539 Free PMC article. - Laboratory testing for respiratory viruses for the clinician.
Attaway CC, Wang H. Attaway CC, et al. Cleve Clin J Med. 2024 Sep 4;91(9 suppl 1):S42-S49. doi: 10.3949/ccjm.91.s1.07. Cleve Clin J Med. 2024. PMID: 39231602 Review. - Role of cell culture for virus detection in the age of technology.
Leland DS, Ginocchio CC. Leland DS, et al. Clin Microbiol Rev. 2007 Jan;20(1):49-78. doi: 10.1128/CMR.00002-06. Clin Microbiol Rev. 2007. PMID: 17223623 Free PMC article. Review.
Cited by
- Detection of respiratory viruses in gargle specimens of healthy children.
Morikawa S, Hiroi S, Kase T. Morikawa S, et al. J Clin Virol. 2015 Mar;64:59-63. doi: 10.1016/j.jcv.2015.01.006. Epub 2015 Jan 13. J Clin Virol. 2015. PMID: 25728080 Free PMC article. - Viral and bacterial interactions in the upper respiratory tract.
Bosch AA, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D. Bosch AA, et al. PLoS Pathog. 2013 Jan;9(1):e1003057. doi: 10.1371/journal.ppat.1003057. Epub 2013 Jan 10. PLoS Pathog. 2013. PMID: 23326226 Free PMC article. Review. - Viral Pneumonia Requiring Differentiation from Acute and Progressive Diffuse Interstitial Lung Diseases.
Ishiguro T, Kobayashi Y, Uozumi R, Takata N, Takaku Y, Kagiyama N, Kanauchi T, Shimizu Y, Takayanagi N. Ishiguro T, et al. Intern Med. 2019;58(24):3509-3519. doi: 10.2169/internalmedicine.2696-19. Epub 2019 Dec 15. Intern Med. 2019. PMID: 31839671 Free PMC article. - Clinical characteristics and outcomes of patients with severe acute respiratory infections (SARI): results from the Egyptian surveillance study 2010-2014.
Hatem A, Mohamed S, Abu Elhassan UE, Ismael EAM, Rizk MS, El-Kholy A, El-Harras M. Hatem A, et al. Multidiscip Respir Med. 2019 Apr 1;14:11. doi: 10.1186/s40248-019-0174-7. eCollection 2019. Multidiscip Respir Med. 2019. PMID: 30976418 Free PMC article. - Epidemiology of respiratory viruses in Saudi Arabia: toward a complete picture.
Farrag MA, Hamed ME, Amer HM, Almajhdi FN. Farrag MA, et al. Arch Virol. 2019 Aug;164(8):1981-1996. doi: 10.1007/s00705-019-04300-2. Epub 2019 May 28. Arch Virol. 2019. PMID: 31139937 Free PMC article. Review.
References
- Brandt C. D., et al. 1969. Infections in 18,000 infants and children in a controlled study of respiratory tract disease. I. Adenovirus pathogenicity in relation to serologic type and illness syndrome. Am. J. Epidemiol. 90:484–500 - PubMed
- Carrat F., et al. 2008. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am. J. Epidemiol. 167:775–785 - PubMed
- Eichner H., Behbehani A. A., Hochstrasser K. 1983. Diagnostic value of nasal secretions, current state: normal values. Laryngol. Rhinol. Otol. (Stuttg.) 52:561–565 (In German.) - PubMed
- Falsey A. R., Criddle M. C., Walsh E. E. 2006. Detection of respiratory syncytial virus and human metapneumovirus by reverse transcription polymerase chain reaction in adults with and without respiratory illness. J. Clin. Virol. 35:46–50 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical