Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors - PubMed (original) (raw)
Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors
Shaomin Li et al. J Neurosci. 2011.
Abstract
In Alzheimer's disease (AD), dementia severity correlates strongly with decreased synapse density in hippocampus and cortex. Numerous studies report that hippocampal long-term potentiation (LTP) can be inhibited by soluble oligomers of amyloid β-protein (Aβ), but the synaptic elements that mediate this effect remain unclear. We examined field EPSPs and whole-cell recordings in wild-type mouse hippocampal slices. Soluble Aβ oligomers from three distinct sources (cultured cells, AD cortex, or synthetic peptide) inhibited LTP, and this was prevented by the selective NR2B inhibitors ifenprodil and Ro 25-6981. Soluble Aβ enhanced NR2B-mediated NMDA currents and extrasynaptic responses; these effects were mimicked by the glutamate reuptake inhibitor dl-threo-β-benzyloxyaspartic acid. Downstream, an Aβ-mediated rise in p38 mitogen-activated protein kinase (MAPK) activation was followed by downregulation of cAMP response element-binding protein, and LTP impairment was prevented by inhibitors of p38 MAPK or calpain. Thus, soluble Aβ oligomers at low nanomolar levels present in AD brain increase activation of extrasynaptic NR2B-containing receptors, thereby impairing synaptic plasticity.
Figures
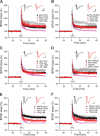
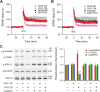
Figure 1.
Soluble Aβ impairment of LTP in the CA1 region of hippocampus requires NR2B-containing NMDA receptors. A, 7PA2 CM rich in soluble Aβ inhibited LTP (pink tracing) induced by high-frequency stimulation (HFS, arrow), but this was restored by the NR2B antagonist, ifenprodil (Ifen) (3 μ
m
) (red circles, n = 7) to its control level (black squares, n = 8). CHO− CM had no effect on LTP (gray tracing). Pink and gray tracings are basic data from just 7PA2 CM or CHO− CM. B, Soluble Aβ impaired-LTP was also rescued by another NR2B antagonist, Ro 25-6981 (Ro) 1 μ
m
, n = 7). C, Soluble Aβ-rich TBS extracts of AD brains (AD_TBS) similarly impaired HFS-induced LTP, but this was restored by ifenprodil (3 μ
m
) (red circles, n = 12) to its control level (black squares, n = 8). D, Synthetic human Aβ S26C dimers (10 n
m
) impaired LTP, but this was restored by ifenprodil (3 μ
m
) (red circles, n = 7) to its control level (black squares, n = 7). E, A low dose of the relative NR2A antagonist PPPA (0.5 μ
m
) alone had no effect on LTP (administered in CHO− CM) (black circles, n = 7) and failed to restore LTP impaired by 7PA2 CM (red triangles, n = 7). Pink and gray tracings are basic data from just 7PA2 CM or CHO− CM. F, A NR2C/NR2D selective antagonist PPDA (1 μ
m
), had no significant effect on the HFS-LTP (black circles, n = 6), and it failed to fully restore Aβ-impaired LTP (red triangles, n = 6). All the NMDA receptor selective antagonists were applied 10 min before Aβ was added. Inset traces are typical field EPSPs recorded before (gray or orange) and after (black or red) HFS for each condition. Horizontal calibration bars, 10 ms; vertical bars, 0.5 mV.
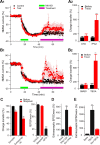
Figure 2.
Soluble Aβ oligomers enhance extrasynaptic NMDA currents in the hippocampus. A, Extrasynaptic NMDA currents were recorded when evoked NMDA EPSCs were isolated from synaptic currents by MK801 treatment (20 μ
m
, green bar) followed by wash out. The magenta bars in A1 and B1 represent the application of either 7PA2 CM versus CHO− CM (A1) or TBOA versus DMSO (B1) treatments. A 2 and B2 are the summary data for the extrasynaptic responses before (black) and 30 min after (red) treatments (Treat). B, Extrasynaptic NMDA currents were also increased after administering the glutamate reuptake inhibitor TBOA (30 μ
m
, green bar) (B1); summary data shown in B2. C, Effects of NR2B antagonist ifenprodil (3 μ
m
) on isolated synaptic and extrasynaptic NMDAR-mediated EPSCs or on NMDAR synaptic fEPSPs in low Mg2+ (0.1 m
m
) ACSF plus NBQX (10 μ
m
) and bicuculline (20 μ
m
). D, Isolated extrasynaptic NMDA currents in 7PA2 CM alone or 7PA2 CM plus Ro 25-6981 (Ro). E, TBOA significantly increased extrasynaptic NMDA currents, which were mostly inhibited by the NR2B antagonist Ro 25-6981 (1 μ
m
). Error bars are ± SEM. *p < 0.05, **p < 0.01.
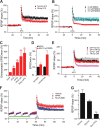
Figure 3.
Soluble Aβ impairs LTP via increased extracellular glutamate levels in the Schaffer collateral-CA1 pathway of hippocampus. A, In contrast with the conventional high-frequency stimulation (two trains of 100 Hz, 1 s, separated by 20 s at test stimulus intensity, black squares, n = 6), a strong HFS (at 1.5× test stimulus intensity) significantly reduces the LTP induction (red circles, n = 5). B, A low dose of NMDA (0.5 μ
m
, red circles, n = 7) prevented HFS-induced hippocampal LTP. C, Extrasynaptic NMDA current changes induced by conventional HFS (HFS1), strong HFS (HFS2), 7PA2 CM, or 7PA2 CM plus HFS (7PA2+HFS). D, Responses of isolated NMDAR-mediated synaptic and extrasynaptic (extrasynap) EPSCs to the 0.5 μ
m
NMDA. E, An enzymatic glutamate scavenger system (GPT plus pyruvate) has no significant effect on normal LTP in CHO− CM (black squares, n = 7) but fully prevented the 7PA2 CM impairment of LTP (red circles, n = 8). F, Three repetitive 5 min episodes of subthreshold, low-frequency stimulation (0.5 Hz) (gray bar) desensitize the synaptic NMDAR effect on LTP induction. Green bar represents the time of application of either NR2A-selective (red squares) or NR2B-selective (blue triangles) antagonists given to prevent the desensitization. G, Summary data for the effect of the NMDAR desensitization regulator, glycine (Gly) (100 μ
m
), on Aβ-impaired LTP. Error bars represent ± SEM. *p < 0.05, **p < 0.01.
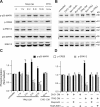
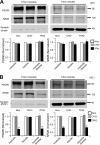
Figure 4.
Involvement of NR2B-containing NMDA receptors in Aβ-induced phosphorylation of p38 MAPK and dephosphorylation of p42/44 MAPK and CREB. A, B, Western blots of the indicated phosphoproteins (with p- prefix) in cultured hippocampal neurons. C, D, Summary histograms quantifying relative immunoreactivity. A, C, Neurons were untreated or treated with 7PA2 CM for 1, 3, 6, or 16 h or with CHO− CM for 16 h. B, D, Hippocampal neurons were exposed as indicated to 7PA2 CM or CHO− CM without or with the NR2B-selective NMDAR inhibitors ifenprodil (Ifen) and Ro 25-6981 (Ro) for 6 h and then analyzed with the indicated antibodies. Data (means ± SEM) are from at least four independent batches of cultured neurons. Error bars represent ± SEM. *p < 0.05, **p < 0.01.
Figure 5.
Soluble Aβ oligomers do not cause detectable synaptic and extrasynaptic fraction changes upon brief exposure, while the synaptic proteins significant decrease after long treatment. A, B, Western blots of biochemically separated synaptic (triton insoluble) versus extrasynaptic (Triton X-100 soluble) fractions from acute hippocampal slices after no treatment (Non) or treatment with CHO− CM or 7PA2 CM for 30 min (A) or 6 h (B). The graphs show quantification of Western blot signals obtained after the three treatments. Error bars represent ± SEM. *p < 0.05, **p < 0.01.
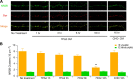
Figure 6.
Soluble Aβ oligomers do not cause detectable synaptic and extrasynaptic morphological changes upon brief exposure. A, Representative photomicrographs of neuronal cultures labeled with antibodies to NR2B (green) or synapsin (Syn) (red). NR2B-positive clusters on dendrites were classified as synaptic or extrasynaptic based on whether they colocalized (yellow in Merge) or not (green in Merge) with synapsin. B, Bar graphs: quantification after 0, 1, 3, 6, or 16 h of exposure to 7PA2 CM or CHO− CM. Scale bar, 10 μm.
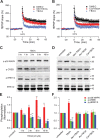
Figure 7.
LTP impairment by soluble Aβ involves the p38 MAPK and calpain signaling pathway. A, p38 MAPK inhibitor SB203580 (5 μ
m
) had no effect on normal LTP (black squares, n = 8) but significantly prevented the inhibition of LTP by 7PA2 CM (red circles, n = 7). B, Calpain selective inhibitor calpeptin (100 μ
m
) slightly depressed LTP (black squares, n = 7), but prevented LTP inhibition by 7PA2 CM (red circles, n = 6). C, Increased phosphorylation (p- prefix) of p38 MAPK and dephosphorylation of ERK1/2 and CREB by 6 h treatment with 7PA2 CM were blocked by calpeptin. SB203580 and calpeptin were applied 20 min before soluble Aβ was added. Error bars represent ± SEM. *p < 0.05, **p < 0.01.
Figure 8.
Involvement of NR2B-containing NMDA receptors in TBOA-induced phosphorylation of p38 MAPK and dephosphorylation of ERK1/2 and CREB. A, p38 MAPK inhibitor, SB 203580 (SB) (5 μ
m
), significantly decreased LTP inhibition by the glutamate reuptake inhibitor TBOA (15 μ
m
). B, Calpeptin (Cal) (100 μ
m
), significantly decreased LTP inhibition by TBOA (15 μ
m
). C, D, Western blots of the indicated phosphoproteins (p- prefix) in cultured hippocampal neurons. E, F, Summary histograms quantifying relative immunoreactivity. C, E, Neurons were untreated or treated with TBOA (20 μ
m
) for 1, 3, 6 or 16 h or without TBOA for 16 h. D, F, Hippocampal neurons were exposed as indicated to TBOA without or with the NR2B-selective NMDAR inhibitors ifenprodil (Ifen) and Ro-6981 (Ro) (0.5 μ
m
) or calpain inhibitor calpeptin (100 μ
m
) for 6 h and then analyzed with the indicated antibodies. Data (means ± SEM) are from at least four independent batches of cultured neurons.
Similar articles
- Enhancement of long-term depression by soluble amyloid β protein in rat hippocampus is mediated by metabotropic glutamate receptor and involves activation of p38MAPK, STEP and caspase-3.
Chen X, Lin R, Chang L, Xu S, Wei X, Zhang J, Wang C, Anwyl R, Wang Q. Chen X, et al. Neuroscience. 2013 Dec 3;253:435-43. doi: 10.1016/j.neuroscience.2013.08.054. Epub 2013 Sep 5. Neuroscience. 2013. PMID: 24012839 - Bioactive human Alzheimer brain soluble Aβ: pathophysiology and therapeutic opportunities.
Li S, Stern AM. Li S, et al. Mol Psychiatry. 2022 Aug;27(8):3182-3191. doi: 10.1038/s41380-022-01589-5. Epub 2022 Apr 28. Mol Psychiatry. 2022. PMID: 35484241 Review. - Magnesium in Alzheimer’s disease.
Chui D, Chen Z, Yu J, Zhang H, Wang W, Song Y, Yang H; Liang Zhou. Chui D, et al. In: Vink R, Nechifor M, editors. Magnesium in the Central Nervous System [Internet]. Adelaide (AU): University of Adelaide Press; 2011. In: Vink R, Nechifor M, editors. Magnesium in the Central Nervous System [Internet]. Adelaide (AU): University of Adelaide Press; 2011. PMID: 29920010 Free Books & Documents. Review.
Cited by
- Calpain-Mediated Degradation of Drebrin by Excitotoxicity In vitro and In vivo.
Chimura T, Launey T, Yoshida N. Chimura T, et al. PLoS One. 2015 Apr 23;10(4):e0125119. doi: 10.1371/journal.pone.0125119. eCollection 2015. PLoS One. 2015. PMID: 25905636 Free PMC article. - Insights into the Pathophysiology of Psychiatric Symptoms in Central Nervous System Disorders: Implications for Early and Differential Diagnosis.
Menculini G, Chipi E, Paolini Paoletti F, Gaetani L, Nigro P, Simoni S, Mancini A, Tambasco N, Di Filippo M, Tortorella A, Parnetti L. Menculini G, et al. Int J Mol Sci. 2021 Apr 23;22(9):4440. doi: 10.3390/ijms22094440. Int J Mol Sci. 2021. PMID: 33922780 Free PMC article. Review. - Sex and gonadal hormones in mouse models of Alzheimer's disease: what is relevant to the human condition?
Dubal DB, Broestl L, Worden K. Dubal DB, et al. Biol Sex Differ. 2012 Nov 5;3(1):24. doi: 10.1186/2042-6410-3-24. Biol Sex Differ. 2012. PMID: 23126652 Free PMC article. - Amyloid-β acts as a regulator of neurotransmitter release disrupting the interaction between synaptophysin and VAMP2.
Russell CL, Semerdjieva S, Empson RM, Austen BM, Beesley PW, Alifragis P. Russell CL, et al. PLoS One. 2012;7(8):e43201. doi: 10.1371/journal.pone.0043201. Epub 2012 Aug 15. PLoS One. 2012. PMID: 22905234 Free PMC article. - Research Progress on the Mechanisms of Protocatechuic Acid in the Treatment of Cognitive Impairment.
Liang S, Zhao Z, Liu L, Zhang Y, Liu X. Liang S, et al. Molecules. 2024 Oct 6;29(19):4724. doi: 10.3390/molecules29194724. Molecules. 2024. PMID: 39407652 Free PMC article. Review.
References
- Berberich S, Jensen V, Hvalby Ø, Seeburg PH, Köhr G. The role of NMDAR subtypes and charge transfer during hippocampal LTP induction. Neuropharmacology. 2007;52:77–86. - PubMed
- Chen QS, Wei WZ, Shimahara T, Xie CW. Alzheimer amyloid beta-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiol Learn Mem. 2002;77:354–371. - PubMed
- Chong YH, Shin YJ, Lee EO, Kayed R, Glabe CG, Tenner AJ. ERK1/2 activation mediates Aβ oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J Biol Chem. 2006;281:20315–20325. - PubMed
- Dewachter I, Filipkowski RK, Priller C, Ris L, Neyton J, Croes S, Terwel D, Gysemans M, Devijver H, Borghgraef P, Godaux E, Kaczmarek L, Herms J, Van Leuven F. Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiol Aging. 2009;30:241–256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials