Functional organization of the thalamic input to the thalamic reticular nucleus - PubMed (original) (raw)
Functional organization of the thalamic input to the thalamic reticular nucleus
Ying-Wan Lam et al. J Neurosci. 2011.
Abstract
Most axons connecting the thalamus and cortex in both directions pass through the thalamic reticular nucleus (TRN), a thin layer of GABAergic cells adjacent to the thalamus, and innervate neurons there. The TRN, therefore, is in a strategic location to regulate thalamocortical communication. We recorded neurons of the somatosensory region of the TRN in a thalamocortical slice preparation and studied the spatial organization of their thalamic input using laser scanning photostimulation. We show that the thalamoreticular pathway is organized topographically for most neurons. The somatosensory region of the TRN can be organized into three tiers. From the inner (thalamoreticular) border to the outer, in a manner roughly reciprocal to the reticulothalamic pathway, each of these tiers receives its input from one of the somatosensory relays of the thalamus--the posterior medial, ventroposterior medial, and ventroposterior lateral nuclei. What is surprising is that approximately a quarter of the recorded neurons received input from multiple thalamic regions usually located in different nuclei. These neurons distribute evenly throughout the thickness of the TRN. Our results, therefore, suggest that there exist a subpopulation of TRN neurons that receive convergent inputs from multiple thalamic sources and engage in more complex patterns of inhibition of relay cells. We propose these neurons enable the TRN to act as an externally driven "searchlight" that integrates cortical and subcortical inputs and then inhibits or disinhibits specific thalamic relay cells, so that appropriate information can get through the thalamus to the cortex.
Figures
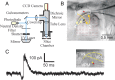
Figure 1.
Experimental methods. A, Schematic diagram of optical setup. B, Photomicrograph of the thalamocortical slice preparation taken during one experiment with a diagram superimposed showing the photostimulation pattern. The star indicates the recording site; red circles indicate locations where the laser was focused during mapping. The locations were stimulated in a distributed manner to maximize the spatial distance between consecutive trials. The positions of the first five trials are indicated (see Materials and Methods for details). C, Control showing IPSCs clearly visible with the holding potential (−40 or −45 mV) used in our experiments. We recorded from a thalamic relay cell in the VPM (red star); inset shows a photomicrograph taken during the recording. The trace shows a large disynaptic IPSC response evoked through the thalamo-reticulo-thalamic neuronal arc when we stimulated near the soma (yellow circle). The cell was held at −40 mV and the laser power was 24 mW. The yellow dotted lines indicate the borders between thalamic nuclei.
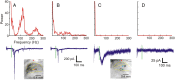
Figure 2.
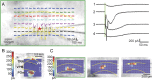
Temporal properties of the responses evoked by photostimulation. A, B, Responses that consist of large, inward spikes riding on top of slow, inward currents (bottom, blue traces). Their power spectra (top, red curves), obtained using Fourier transforms, indicate the presence of high-frequency components. C, A response that consists only of a small, slow, inward current (bottom, blue trace) and its power spectrum (top, red curve). D, Recording from a trial in which photostimulation did not evoke any response. Insets, Photomicrographs taken during experiments. Green vertical lines indicate the timing of photostimulation. Recording and stimulation sites are indicated by stars and circles, respectively. Laser power was 31 mW in A and B, 56 mW in C and D. Holding potential was −40 mV.
Figure 3.
Inward spikes evoked by photostimulation were not blocked by sodium channel blocker. Traces show the responses of a TRN neuron to photostimulation at different holding potentials. The vertical lines indicate the timing of the laser pulses. The recording electrode contained the Cs+ pipette solution and 5 m
m
QX-314, a sodium channel blocker. Inset shows a photomicrograph taken during the experiment; the star and circle indicate the recording and stimulation sites, respectively. Power of the laser was 80 mW.
Figure 4.
Responses of TRN neurons to thalamic photostimulation. A, Example of photostimulation of VPM evoking large, narrow EPSC spikes riding on top of a slow depolarization (1, 2); photostimulation of POm elicited slow depolarization (3, 4). The response traces to photostimulation of 256 locations are overlaid on top of a photomicrograph taken during the experiment and placed where they were evoked. The red star indicates the recording site and the yellow dotted lines indicate the borders between thalamic nuclei. The color map insets show the extent of the footprints more clearly by indicating where the power of the response traces exceeds threshold. B, Four selected traces (1–4) shown on a larger scale. C, Power spectra of three selected traces (1, 2, and 4) and one where no response was evoked (5), as calculated by Fourier analysis. The laser power used was 40 mW and the holding potential was −40 mV.
Figure 5.
Thalamic input footprints to TRN neurons. A, B, Example of responses evoked from a single area in VPM. A, Responses to photostimulation overlaid on top of photomicrograph taken during the experiment in a similar format as in Figure 2. The red star indicates the recording site. B, Left, The color map indicates where power of the responses exceed threshold overlaid on the photomicrograph of the thalamocortical slices; right, recordings from the area where photostimulation-evoked responses are enlarged (green rectangle). Yellow dotted lines indicate the border between nuclei. Laser power used was 24 mW. C, D, Example of responses elicited from two separate areas in the thalamus. C, Responses overlaid on the photomicrograph of the brain slice, in the same format as A. D, Left, The color map indicates where the responses exceed threshold; right, traces shown as enlarged from the areas within the VPM (red rectangle) and POm (green rectangle) where responses could be evoked. Laser power was 60 mW. Holding potential was −40 mV in both experiments.
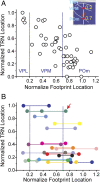
Figure 6.
Relationship between the normalized locations of TRN neurons and their thalamic inputs. A, Relationship between neurons that had only one footprint. Inset illustrates the method for normalizing locations of the input footprints (see Results for details). B, Relationship between neurons that received more than one thalamic input: all received two inputs except for the cell indicated by the arrow, which had three inputs. Footprints from the same neuron are color coded and connected by horizontal lines. A, B, Vertical dotted lines indicate the normalized borders between VPL, VPM, and POm.
Figure 7.
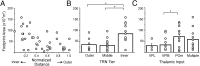
Plots of footprint areas versus the locations of TRN neurons and thalamic input. A, Scatter plot of the footprint areas of TRN neurons versus their normalized distance from the thalamoreticular border. B, Average footprint areas of the TRN neurons located in the three tiers of the TRN. TRN neurons are divided into three groups according to the tier in which they are located (see Matherials and Methods). Circles indicate results of individual experiments. C, Average footprint areas of TRN neurons with different thalamic inputs. Cells are divided into four groups according to the origin of their thalamic input (see Materials and Methods). Circles represent the results from individual experiments. *, Significant differences (p < 0.05) in a post hoc analysis using Fisher's PLSD. Bars indicate group averages; error bars are SEMs.
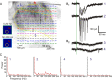
Figure 8.
Estimates of shape and size of dendritic arbors of TRN neurons using photostimulation. A, Responses of TRN neuron (red star) to direct photostimulation. The neuron was stimulated at 128 locations, as indicated. Recordings immediately after the laser pulse are overlaid on a photomicrograph of the slice. Yellow dotted lines indicate the borders of the TRN. Right, Four numbered traces were selected and are displayed on a larger scale. B, Pseudocolor plot of the thalamic input to the neuron in A. Peak EPSCs are plotted using the indicated color scale and overlaid on a photomicrograph of the slice. Borders between thalamic nuclei are indicated with yellow dotted lines. Laser power was 70 mW and the holding potential used during recording was −40 mV. C, Left, Pseudocolor plot of the peak inward (depolarization) currents averaged from two maps for the cell shown in A. Middle and right, Analogous pseudocolor plots for two other TRN neurons. Laser power used for all three experiments was 14 mW and all cells were all held at −45 mV during the recording.
Figure 9.
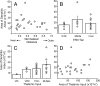
A, Scatter plot of area of the dendritic arbor versus normalized distance from thalamoreticular border. B, Average dendritic arbor areas for TRN neurons located in the three tiers of TRN. C, Plot of average dendritic arbor areas versus the origin of thalamic input. Neurons are grouped according to the nucleus of each of their thalamic inputs. D, Scatter plot of the areas of the dendritic arbor of TRN neurons versus the size of their thalamic input. Error bars represent SEMs; circles indicate individual cells.
Figure 10.
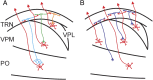
Schematic diagram showing the spatial organization of the thalamoreticular pathway. A, A subtype of neurons that receive input from and project back to the somatosensory thalamus in a topographic manner. B, A subtype of neurons that receive convergent inputs from two or more thalamic nuclei and output topographically back to the thalamus.
Similar articles
- Functional topographic organization of the motor reticulothalamic pathway.
Lam YW, Sherman SM. Lam YW, et al. J Neurophysiol. 2015 May 1;113(9):3090-7. doi: 10.1152/jn.00847.2014. Epub 2015 Feb 25. J Neurophysiol. 2015. PMID: 25717161 Free PMC article. - Different topography of the reticulothalmic inputs to first- and higher-order somatosensory thalamic relays revealed using photostimulation.
Lam YW, Sherman SM. Lam YW, et al. J Neurophysiol. 2007 Nov;98(5):2903-9. doi: 10.1152/jn.00782.2007. Epub 2007 Sep 19. J Neurophysiol. 2007. PMID: 17881481 - Two dynamically distinct circuits drive inhibition in the sensory thalamus.
Martinez-Garcia RI, Voelcker B, Zaltsman JB, Patrick SL, Stevens TR, Connors BW, Cruikshank SJ. Martinez-Garcia RI, et al. Nature. 2020 Jul;583(7818):813-818. doi: 10.1038/s41586-020-2512-5. Epub 2020 Jul 22. Nature. 2020. PMID: 32699410 Free PMC article. - Thalamic reticular nucleus in the thalamocortical loop.
Takata N. Takata N. Neurosci Res. 2020 Jul;156:32-40. doi: 10.1016/j.neures.2019.12.004. Epub 2019 Dec 5. Neurosci Res. 2020. PMID: 31812650 Review. - Thalamic relays and cortical functioning.
Sherman SM. Sherman SM. Prog Brain Res. 2005;149:107-26. doi: 10.1016/S0079-6123(05)49009-3. Prog Brain Res. 2005. PMID: 16226580 Review.
Cited by
- Laminarly orthogonal excitation of fast-spiking and low-threshold-spiking interneurons in mouse motor cortex.
Apicella AJ, Wickersham IR, Seung HS, Shepherd GM. Apicella AJ, et al. J Neurosci. 2012 May 16;32(20):7021-33. doi: 10.1523/JNEUROSCI.0011-12.2012. J Neurosci. 2012. PMID: 22593070 Free PMC article. - Differential gating of thalamocortical signals by reticular nucleus of thalamus during locomotion.
Marlinski V, Sirota MG, Beloozerova IN. Marlinski V, et al. J Neurosci. 2012 Nov 7;32(45):15823-36. doi: 10.1523/JNEUROSCI.0782-12.2012. J Neurosci. 2012. PMID: 23136421 Free PMC article. - Anatomical pathways involved in generating and sensing rhythmic whisker movements.
Bosman LW, Houweling AR, Owens CB, Tanke N, Shevchouk OT, Rahmati N, Teunissen WH, Ju C, Gong W, Koekkoek SK, De Zeeuw CI. Bosman LW, et al. Front Integr Neurosci. 2011 Oct 4;5:53. doi: 10.3389/fnint.2011.00053. eCollection 2011. Front Integr Neurosci. 2011. PMID: 22065951 Free PMC article. - Activation of both Group I and Group II metabotropic glutamatergic receptors suppress retinogeniculate transmission.
Lam YW, Sherman SM. Lam YW, et al. Neuroscience. 2013 Jul 9;242:78-84. doi: 10.1016/j.neuroscience.2013.03.043. Epub 2013 Apr 1. Neuroscience. 2013. PMID: 23558090 Free PMC article. - Sleep spindles in humans: insights from intracranial EEG and unit recordings.
Andrillon T, Nir Y, Staba RJ, Ferrarelli F, Cirelli C, Tononi G, Fried I. Andrillon T, et al. J Neurosci. 2011 Dec 7;31(49):17821-34. doi: 10.1523/JNEUROSCI.2604-11.2011. J Neurosci. 2011. PMID: 22159098 Free PMC article.
References
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
- Canepari M, Nelson L, Papageorgiou G, Corrie JE, Ogden D. Photochemical and pharmacological evaluation of 7-nitroindolinyl-and 4-methoxy-7-nitroindolinyl-amino acids as novel, fast caged neurotransmitters. J Neurosci Methods. 2001;112:29–42. - PubMed
- Cox CL, Sherman SM. Control of dendritic outputs of inhibitory interneurons in the lateral geniculate nucleus. Neuron. 2000;27:597–610. - PubMed
- Crabtree JW, Collingridge GL, Isaac JT. A new intrathalamic pathway linking modality-related nuclei in the dorsal thalamus. Nat Neurosci. 1998;1:389–394. - PubMed
Publication types
MeSH terms
Grants and funding
- R03 NS058468-01/NS/NINDS NIH HHS/United States
- R01 DC008794/DC/NIDCD NIH HHS/United States
- R03 NS058468/NS/NINDS NIH HHS/United States
- R03 NS058468-02/NS/NINDS NIH HHS/United States
- EY-03038/EY/NEI NIH HHS/United States
- NS-058468/NS/NINDS NIH HHS/United States
- DC-008794/DC/NIDCD NIH HHS/United States
- R01 EY003038/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources