Three-dimensional structure and catalytic mechanism of cytosine deaminase - PubMed (original) (raw)
. 2011 Jun 7;50(22):5077-85.
doi: 10.1021/bi200483k. Epub 2011 May 12.
Affiliations
- PMID: 21545144
- PMCID: PMC3107989
- DOI: 10.1021/bi200483k
Three-dimensional structure and catalytic mechanism of cytosine deaminase
Richard S Hall et al. Biochemistry. 2011.
Abstract
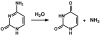
Cytosine deaminase (CDA) from E. coli is a member of the amidohydrolase superfamily. The structure of the zinc-activated enzyme was determined in the presence of phosphonocytosine, a mimic of the tetrahedral reaction intermediate. This compound inhibits the deamination of cytosine with a K(i) of 52 nM. The zinc- and iron-containing enzymes were characterized to determine the effect of the divalent cations on activation of the hydrolytic water. Fe-CDA loses activity at low pH with a kinetic pK(a) of 6.0, and Zn-CDA has a kinetic pK(a) of 7.3. Mutation of Gln-156 decreased the catalytic activity by more than 5 orders of magnitude, supporting its role in substrate binding. Mutation of Glu-217, Asp-313, and His-246 significantly decreased catalytic activity supporting the role of these three residues in activation of the hydrolytic water molecule and facilitation of proton transfer reactions. A library of potential substrates was used to probe the structural determinants responsible for catalytic activity. CDA was able to catalyze the deamination of isocytosine and the hydrolysis of 3-oxauracil. Large inverse solvent isotope effects were obtained on k(cat) and k(cat)/K(m), consistent with the formation of a low-barrier hydrogen bond during the conversion of cytosine to uracil. A chemical mechanism for substrate deamination by CDA was proposed.
Figures
Figure 1
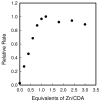
Reconstitution of apo-CDA (8.5 μM) with varying equivalents of ZnCl2. The catalytic activity was determined after dilution of the enzyme to 50 nM using 2.0 mM cytosine in 50 mM TRIS, pH 7.5 and 30 °C.
Figure 2
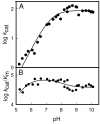
pH-rate profiles for the deamination of cytosine by Zn-CDA. (A) log _k_cat vs. pH profile for Zn-CDA. The solid line represents a fit of the data with equation 2. (B) log _k_cat/_K_m vs pH profile for Zn-CDA. The solid line represents a fit of the data with equation 3.
Figure 3
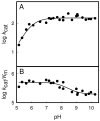
pH-rate profiles for the deamination of cytosine by Fe-CDA. (A) log _k_cat vs pH profile for Fe-CDA. The solid line represents a fit of the data with equation 2. (B) log _k_cat/_K_m vs pH profile for Fe-CDA. The solid line represents a fit of the data with equation 4.
Figure 4
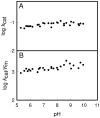
pH-rate profiles for the deamination of cytosine by Zn-H246Q CDA. (A) log _k_cat vs pH profile for Zn-H246Q CDA. (B) log _k_cat/_K_m vs pH profile for Zn-H246Q CDA.
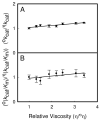
Figure 5
(A) Viscosity effects on the relative values of _k_cat using sucrose as the micro-viscogen at pH 9.0. (B) Viscosity effects on the relative values of _k_cat/_K_m for Zn-CDA using sucrose as the micro-viscogen at pH 9.0.
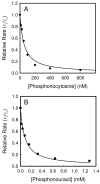
Figure 6
Inhibition of Zn-CDA in 50 mM TRIS, pH 7.5, with (A) phosphonocytosine (5) and (B) phosphonouracil (6). Solid lines represent fits of the data with equation 5. Enzyme and inhibitor solutions were pre-incubated together for 30 minutes at 30 °C prior to initiating the reactions with 0.2 mM cytosine.
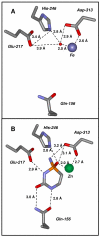
Figure 7
Structure of the active site of cytosine deaminase. (A) Fe-CDA in the absence of bound ligands. The coordinates were taken from PDB code: 1K6W. The three residues that facilitate the nucleophilic attack of water (Glu-217, His-246, and Asp-313) and the residue that facilitates the binding of cytosine (Gln-156) are shown. (B) The active site of Zn-CDA in the presence of the tight-binding transition state inhibitor phosphonocytosine (PDB code: 3O7U).
Figure 8
The active site of Zn-CDA with bound phosphonocytosine. Omit electron density map (Fo-Fc) is contoured at 5.0 sigma. The ligand was omitted from the model and the remainder of the unit cell was subjected to a cycle of simulated annealing with PHENIX at 3000 degrees C.
Scheme 1
Scheme 2
Scheme 3
Scheme 4
Similar articles
- Reaction mechanism of zinc-dependent cytosine deaminase from Escherichia coli: a quantum-chemical study.
Manta B, Raushel FM, Himo F. Manta B, et al. J Phys Chem B. 2014 May 29;118(21):5644-52. doi: 10.1021/jp501228s. Epub 2014 May 15. J Phys Chem B. 2014. PMID: 24833316 - Rescue of the orphan enzyme isoguanine deaminase.
Hitchcock DS, Fedorov AA, Fedorov EV, Dangott LJ, Almo SC, Raushel FM. Hitchcock DS, et al. Biochemistry. 2011 Jun 28;50(25):5555-7. doi: 10.1021/bi200680y. Epub 2011 Jun 7. Biochemistry. 2011. PMID: 21604715 Free PMC article. - Role of glutamate 64 in the activation of the prodrug 5-fluorocytosine by yeast cytosine deaminase.
Wang J, Sklenak S, Liu A, Felczak K, Wu Y, Li Y, Yan H. Wang J, et al. Biochemistry. 2012 Jan 10;51(1):475-86. doi: 10.1021/bi201540z. Epub 2011 Dec 29. Biochemistry. 2012. PMID: 22208667 Free PMC article. - Catalytic mechanism of yeast cytosine deaminase: an ONIOM computational study.
Sklenak S, Yao L, Cukier RI, Yan H. Sklenak S, et al. J Am Chem Soc. 2004 Nov 17;126(45):14879-89. doi: 10.1021/ja046462k. J Am Chem Soc. 2004. PMID: 15535715 - Evaluation of molecular models for the affinity maturation of antibodies: roles of cytosine deamination by AID and DNA repair.
Samaranayake M, Bujnicki JM, Carpenter M, Bhagwat AS. Samaranayake M, et al. Chem Rev. 2006 Feb;106(2):700-19. doi: 10.1021/cr040496t. Chem Rev. 2006. PMID: 16464021 Free PMC article. Review. No abstract available.
Cited by
- X-Ray Structure and Mutagenesis Studies of the N-Isopropylammelide Isopropylaminohydrolase, AtzC.
Balotra S, Warden AC, Newman J, Briggs LJ, Scott C, Peat TS. Balotra S, et al. PLoS One. 2015 Sep 21;10(9):e0137700. doi: 10.1371/journal.pone.0137700. eCollection 2015. PLoS One. 2015. PMID: 26390431 Free PMC article. - Structure of N-formimino-L-glutamate iminohydrolase from Pseudomonas aeruginosa.
Fedorov AA, Martí-Arbona R, Nemmara VV, Hitchcock D, Fedorov EV, Almo SC, Raushel FM. Fedorov AA, et al. Biochemistry. 2015 Jan 27;54(3):890-7. doi: 10.1021/bi501299y. Epub 2015 Jan 16. Biochemistry. 2015. PMID: 25559274 Free PMC article. - Nucleobase deaminases: a potential enzyme system for new therapies.
Gaded V, Anand R. Gaded V, et al. RSC Adv. 2018 Jun 28;8(42):23567-23577. doi: 10.1039/c8ra04112a. eCollection 2018 Jun 27. RSC Adv. 2018. PMID: 35540270 Free PMC article. Review. - Engineered deaminases as a key component of DNA and RNA editing tools.
Budzko L, Hoffa-Sobiech K, Jackowiak P, Figlerowicz M. Budzko L, et al. Mol Ther Nucleic Acids. 2023 Oct 20;34:102062. doi: 10.1016/j.omtn.2023.102062. eCollection 2023 Dec 12. Mol Ther Nucleic Acids. 2023. PMID: 38028200 Free PMC article. Review. - Discovery of a bacterial 5-methylcytosine deaminase.
Hitchcock DS, Fedorov AA, Fedorov EV, Almo SC, Raushel FM. Hitchcock DS, et al. Biochemistry. 2014 Dec 2;53(47):7426-35. doi: 10.1021/bi5012767. Epub 2014 Nov 19. Biochemistry. 2014. PMID: 25384249 Free PMC article.
References
- Ireton GC, McDermott G, Black ME, Stoddard BL. The structure of Escherichia coli cytosine deaminase. J. Mol. Biol. 2002;315:687–697. - PubMed
- Seibert CM, Raushel FM. Structural and catalytic diversity within the amidohydrolase superfamily. Biochemistry. 2005;44:6383–6391. - PubMed
- Holm L, Sander C. An evolutionary treasure: unification of a broad set of amidohydrolases related to urease. Proteins. 1997;28:72–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous