Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus - PubMed (original) (raw)
Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus
Juan M Encinas et al. Cell Stem Cell. 2011.
Abstract
Production of new neurons in the adult hippocampus decreases with age; this decline may underlie age-related cognitive impairment. Here we show that continuous depletion of the neural stem cell pool, as a consequence of their division, may contribute to the age-related decrease in hippocampal neurogenesis. Our results indicate that adult hippocampal stem cells, upon exiting their quiescent state, rapidly undergo a series of asymmetric divisions to produce dividing progeny destined to become neurons and subsequently convert into mature astrocytes. Thus, the decrease in the number of neural stem cells is a division-coupled process and is directly related to their production of new neurons. We present a scheme of the neurogenesis cascade in the adult hippocampus that includes a proposed "disposable stem cell" model and accounts for the disappearance of hippocampal neural stem cells, the appearance of new astrocytes, and the age-related decline in the production of new neurons.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
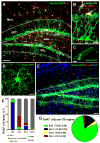
Fig. 1. Nestin-GFP expressing cells and dividing cell populations in the DG
(A, B) Distribution of Nestin-GFP-expressing cells in the DG, after immunostaining for GFP (green) and NG2 (red). Pericytes and oligodendrocyte progenitor cells (OPCs, immunopositive for NG2), are distributed throughout the DG, but are largely excluded from the GCL and the SGZ (outlined). (C, D) High magnification images of a pericyte (C) and an OPC (D). (E-G). Dividing cells in the DG. Nestin-GFP mice (n=4; age 2 months) received 3 injections of BrdU (150mg/kg) at 3 hr intervals and were sacrificed 1hr after the last injection. The number and phenotype of BrdU-positive cells (red) was determined, after staining for GFP (green) and GFAP (blue), for the defined regions of the DG: molecular layer (Mol L), GCL, SGZ, and hilus. Vast majority of dividing cells are located in the SGZ. Outside the SGZ, where QNPs and ANPs account for the largest number of dividing cells, NG2-positive morphologically distinct OPCs are the main proliferating cell type. In rare occasions, BrdU+Nestin-GFP+ pericytes and BrdU+Nestin-GFP- cells (most likely representing endothelial cells) were detected in the blood vessels. Extremely rare BrdU+GFAP+Nestin-GFP- astrocytes were observed only in the hilus and the molecular layer. Scale bars are 50μm in A, 10μm in B, 5μm in C and D., and 75μm in E. See also Figures S1 and S2 and Table S1.
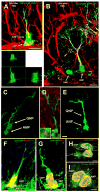
Fig. 2. ANPs are born from QNPs
(A,B) In Gli1-CreER/RCE animals, GFP is expressed exclusively in QNPs 12-18 hr after tamoxifen induction. Later (48 hr after the induction in A), asymmetrically dividing QNPs giving rise to ANPs can be observed; lower panel shows a focal plane from the orthogonal projection of the same pair of cells. Plane of division (dotted line) is often parallel to the SGZ. Furthermore (120 hr after the induction in B), separate ANPs can be identified. (C, D) A pair of QNP and ANP cells in late telophase in the DG of Gli1-CreER/RCE mouse 24 hrs after tamoxifen induction. In D, the midbody is visualized by antibody to Aurora B (arrow; also shown at higher magnification in the inset) to show that such pairs indeed correspond to a newly divided QNP and its daughter cell. (E) A pair of a QNP and an ANP cell in late telophase in the DG of Nestin-CreER/Z/EG mouse 24 hr after tamoxifen induction. (F-I) Pairs of QNP and ANP cells (F, G) and of ANP cells (H, I) in telophase in the DG of Nestin-GFP mice; F and I are z-stack maximum projections (5 and 7μm thick, respectively), G and H are single focal planes (1μm thick). Nuclei of dividing cells are visualized with antibodies to BrdU (24 hr after a single pulse) (F, H) or to phosphorylated histone H3 (G, I). The plane of division (dotted line) is most often parallel to the SGZ for the QNP/ANP pairs but can be different for the ANP pairs. Channels for multiple labels are indicated on the figures. Scale bar is 10μm in A-E and 5μm in F-I. See also Figures S3 and S4.
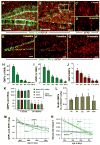
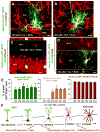
Fig. 3. Age-dependent decrease in the number of hippocampal stem and progenitor cells
(A-D) Nestin-GFP- and GFAP-positive cells in the DG of 1 month old (A, C) and 24 month old (B, D) Nestin-GFP mice. C and D correspond to the boxed regions in A and B, respectively, and show GFAP-positive radial astrocyte-like cells in 1 and 24 months old mice. (E-G) QNP and ANP cells (green nuclei) in the DG of 2, 8, and 24 month old Nestin-CFPnuc mice. Note the increase in the number of GFAP-positive stellar astrocytes with age in A, B, E-G. Color channels are indicated. Scale bar is 50μm in A, B, E-G, and 25μm in C, D. (H-J) Quantification of age-related changes in QNPs (H), ANPs (I), and the QNP/ANP ratio (J) in Nestin-CFPnuc animals (numbers, also presented in Table S2, are given as mean±SEM; at least 4 animals per group). In this and other figures each animal is represented by a dot on the graph (such dots can overlap for close values). (K) Quantification of Nestin-GFP QNPs and radial astrocyte-like cells (RAs) stained for GFAP, nestin, and vimentin in 1 and 24 months old Nestin-GFP animals. (L) Percentage of BrdU-labeled QNPs among all QNP cells in Nestin-GFP mice of different ages after a single pulse of BrdU (150mg/kg, analyzed 24hr later). (M, N) Polynomial fits of the QNP and ANP content (M) and the disappearance (decay) rates (N) of ANPs and QNPs. Dotted lines show a range corresponding to one standard deviation. The solid lines are interpolations. See also Table S2.
Fig. 4. The fate of dividing cells in the DG
(A) Time course of changes in the number of BrdU-positive cells after pulse labeling. 2 month old Nestin-CFPnuc mice (n=4 per time point) received a single injection of BrdU (150 mg/kg) and the number of BrdU-labeled cells in the DG was monitored over the course of 30 days. (B) Rate of changes in the number of BrdU+ cells, reflecting periods of active proliferation, rapid loss, and slower loss of the newborn cells. (C, D) Time course of changes in defined classes of progenitors and mature cells in the DG of Nestin-CFPnuc mice. BrdU+ cells were immunophenotyped to determine the numbers of labeled cells in defined classes. The results for QNPs, ANPs, NBs, mature neurons (granule cells, GC), and stellar astrocytes (Ast) are presented for the total number of cells in each class (C) and their fraction among total BrdU-labeled cells (D). Note the logarithmic y scale in C. (E-H). Differentiation of newborn cells in Nestin-CFPnuc mice. BrdU-labeled QNPs (12 hr after injection of BrdU, E), ANPs and NBs (3 days after injection, F), astrocytes (15 days after injection, G), and mature neurons (30 days after injection, H; shown with orthogonal projections). Color channels are indicated. Scale bar is 10μm. (I-K) Pulse-chase experiment with Nestin-GFP mice. Nestin-GFP mice received a single injection of BrdU (150 mg/kg) and were sacrificed at different time points. At early time points after BrdU injection, all of the BrdU+GFAP+ cells also express Nestin-GFP and have QNP morphology. 10 days after BrdU injection, BrdU+GFAP+ cells lacking Nestin-GFP expression can be observed (I). At this time the morphology of the BrdU+GFAP+ cells has already started to change, with branching of the apical GFAP+ process. 30 days after BrdU injection, none of the BrdU+GFAP+ cells express Nestin-GFP and their morphology resemble that of mature astrocytes (J). Quantification of the time-dependent changes in the number of BrdU+GFAP+ cells (QNPs and astrocytes together), BrdU+GFAP+GFP+ cells (QNPs), and BrdU+GFAP+GFP- cells (astrocytes) in the SGZ and GCL. Note that while the number of BrdU-labeled QNP cells declines, the number of BrdU-labeled GFAP+ cells remains the same. Color channels are indicated. Scale bar is 10μm.
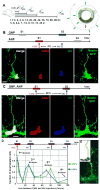
Fig. 5. QNPs undergo division-coupled astrocytic differentiation
(A-D) Recombination in Nestin-CreER/Z/EG mice (3-4 months old) was induced by tamoxifen treatment. Dividing cells were labeled by BrdU. 10 days after tamoxifen induction and BrdU labeling, GFP+BrdU+ QNPs change their morphology, branching their apical radial processes and extending basal cytoplasmic extensions with multiple ramifications (A). 30 days later they extend processes from the soma, show characteristic star-shape morphology and extensive ramification of the branches and become indistinguishable from the surrounding stellar astrocytes (B). GFP+BrdU+NeuN+ granule neurons, detected 30 days after tamoxifen induction and BrdU injection; (C; shown with orthogonal projections). GFP+BrdU+S100β+ mature astrocytes, detected 45 days after tamoxifen induction and BrdU injection (D). Color channels are indicated. Bar is 10μm in A-D. (E) Changes in GFP+BrdU+GFAP+ cells (labeled QNPs and new astrocytes together), GFP+BrdU+S100β+ cells (new mature astrocytes), and GFP+BrdU+NeuN+ cells (new neurons), as fraction of the total number of GFP+BrdU+ cells, after induction with tamoxifen and labeling with BrdU. Note that new mature astrocytes appear only 20 days after the induction and that the fraction of dividing QNPs and astrocytes does not undergo significant changes. Also note that the fractions of neurons and astrocytes among GFP+BrdU+ double-labeled cells is the same as for BrdU+ single-labeled cells in Fig.4. (F) Schematic representation, with a temporal scale, of the changes that QNP undergoes when becoming an astrocyte, with gradual appearance of the apical, basal, and somatic processes. See also Figures S5 and S6.
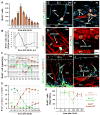
Fig. 6. Dynamics of QNP and ANP division in the DG
(A) General scheme of the double S-phase labeling protocol. Nestin-GFP mice (2 months old, n=3 per time point) received a single injection of CldU followed, at different time interval (t), by a single injection of IdU. Scheme on the right illustrates that a cell marked by CldU injection during the S phase is able to incorporate the second label (IdU) if it is injected during the same S phase but not later, during the G2, M, or G1 phases. However, the cell can become double-labeled if IdU is injected when the CldU-labeled cell again enters the S phase during the second, third, and subsequent rounds of division. (B) An example of double S-phase labeling with the CldU and IdU injections separated by 4 hr. In this example the interval between label injections is not commensurate with the length of the cell cycle of the ANPs or QNPs, and CldU labels the S-phase of the ANP, but not of QNP, whereas IdU labels the S-phase of the QNP, but not of ANP. On the micrograph the nucleus of the ANP cells is labeled with CldU (red) and the nucleus of the QNP cells is labeled with IdU (blue); co-localization of CldU and GFP is yellow. (C) An example of double S-phase labeling with the CdU and IdU injections separated by 28 hr. CldU labels the S-phase of the first division cycle and IdU labels the S-phase of the second cycle (stripped bar). The length of the cell cycle is commensurate with the interval between label injections and some cells have incorporated both labels. On the micrograph the nuclei of a QNP and an ANP cells (distinguished by the presence of a GFP-positive radial process) are labeled with CldU (red) and IdU (blue); triple co-localization is white. Scale bar is 10μm in B, C. (D) Distinct cycles of division can be detected by determining the fraction of CldU/IdU double-labeled QNP and ANP cells at given time intervals. The length of the cell cycles can be determined from the position of the peaks and the length of the S phase can be determined from the slope of the decrease during the first S phase. Both QNPs (green) and ANPs (blue) divide several times in a close succession as suggested by the presence of several peaks in dual labeling (the first peak corresponding to the 0 hr time point); note that the first division also reflects the asymmetric division of the QNP that gives rise to an ANP. (E) A cluster of dividing cells in the SGZ. Several BrdU-labeled ANPs are located next to a BrdU-labeled QNP after 2 hr labeling; such clusters should be observed if QNP, as well as ANPs, reenters the cell cycle but not if it becomes quiescent after giving birth to an ANP (note that this experiment was performed with 6 month old mice, in which progenitor cells undergo a larger number of divisions than in 2 months old animals and in which there are fewer dividing cells such that the clusters of dividing cells are well separated). Inset: schematic representation of the cells and their BrdU-labeled nuclei.
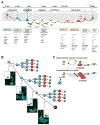
Fig. 7. A schematic summary of differentiation cascade in the DG
(A) A schematic summary of the neuronal and astrocytic differentiation cascade. QNPs generate, through ~3 several asymmetric divisions, the ANPs that, after ~2 rounds of symmetric divisions, exit the cell cycle and become NB1 cells. NB1 cells mature into NB2 and then into INs and differentiated mature neurons; this is accompanied by a massive loss of newborn cells. After a rapid succession of several divisions, QNPs exit the cell cycle and start acquiring the astrocytic morphology. The estimated number of division cycles is presented for a young adult (2-3 months old) mouse and is expected to be different at other ages. Time intervals of the major steps of the cascade are indicated. (B) A scheme of divisions and death of stem cells and their progeny in the DG. Micrographs correspond to the predicted combinations of BrdU-labeled cells after short-term labeling; from top to bottom – first S phase of a QNP; second S phase of a QNP and the first S phase of the daughter ANP; third S phase of a QNP and second S phase of the daughter ANPs; QNP exiting the string of division (unlabeled) and progeny ANPs in the third S phase. Insets: schematic representation of the cells and their BrdU-labeled nuclei. (C) In the conventional “repeated stem cell self-renewal” model, a quiescent stem cell is activated, undergoes an asymmetric division, produces a progeny that eventually differentiates (in this case into a neuron), and returns to the quiescent state to be activated again several times until the death of this stem cell or of the organism. In the “disposable stem cell” model, a stem cell is quiescent for the entire postnatal life, is activated, undergoes several rapid asymmetric divisions producing progeny and quits the pool of stem cells by differentiation (in this case into an astrocyte).
Comment in
- Neural stem cells: disposable, end-state glia?
Lugert S, Taylor V. Lugert S, et al. Cell Stem Cell. 2011 May 6;8(5):464-5. doi: 10.1016/j.stem.2011.04.006. Cell Stem Cell. 2011. PMID: 21549320 No abstract available. - The pessimist's and optimist's views of adult neurogenesis.
Kempermann G. Kempermann G. Cell. 2011 Jun 24;145(7):1009-11. doi: 10.1016/j.cell.2011.06.011. Cell. 2011. PMID: 21703445
Similar articles
- Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis.
Ermini FV, Grathwohl S, Radde R, Yamaguchi M, Staufenbiel M, Palmer TD, Jucker M. Ermini FV, et al. Am J Pathol. 2008 Jun;172(6):1520-8. doi: 10.2353/ajpath.2008.060520. Epub 2008 May 8. Am J Pathol. 2008. PMID: 18467698 Free PMC article. - Immature Neurons and Radial Glia, But Not Astrocytes or Microglia, Are Altered in Adult Cntnap2 and Shank3 Mice, Models of Autism.
Cope EC, Briones BA, Brockett AT, Martinez S, Vigneron PA, Opendak M, Wang SS, Gould E. Cope EC, et al. eNeuro. 2016 Oct 17;3(5):ENEURO.0196-16.2016. doi: 10.1523/ENEURO.0196-16.2016. eCollection 2016 Sep-Oct. eNeuro. 2016. PMID: 27785461 Free PMC article. - Noggin rescues age-related stem cell loss in the brain of senescent mice with neurodegenerative pathology.
Díaz-Moreno M, Armenteros T, Gradari S, Hortigüela R, García-Corzo L, Fontán-Lozano Á, Trejo JL, Mira H. Díaz-Moreno M, et al. Proc Natl Acad Sci U S A. 2018 Nov 6;115(45):11625-11630. doi: 10.1073/pnas.1813205115. Epub 2018 Oct 23. Proc Natl Acad Sci U S A. 2018. PMID: 30352848 Free PMC article. - Age-dependent decline in neurogenesis of the hippocampus and extracellular nucleotides.
Takei Y. Takei Y. Hum Cell. 2019 Apr;32(2):88-94. doi: 10.1007/s13577-019-00241-9. Epub 2019 Feb 7. Hum Cell. 2019. PMID: 30730038 Review. - Modes of division and differentiation of neural stem cells.
Lazutkin A, Podgorny O, Enikolopov G. Lazutkin A, et al. Behav Brain Res. 2019 Nov 18;374:112118. doi: 10.1016/j.bbr.2019.112118. Epub 2019 Jul 29. Behav Brain Res. 2019. PMID: 31369774 Free PMC article. Review.
Cited by
- Environmental enrichment in middle age rats improves spatial and object memory discrimination deficits.
Miranda M, Navas MC, Zanoni Saad MB, Piromalli Girado D, Weisstaub N, Bekinschtein P. Miranda M, et al. Front Behav Neurosci. 2024 Oct 17;18:1478656. doi: 10.3389/fnbeh.2024.1478656. eCollection 2024. Front Behav Neurosci. 2024. PMID: 39494036 Free PMC article. - A herbal medicine for Alzheimer's disease and its active constituents promote neural progenitor proliferation.
Mao J, Huang S, Liu S, Feng XL, Yu M, Liu J, Sun YE, Chen G, Yu Y, Zhao J, Pei G. Mao J, et al. Aging Cell. 2015 Oct;14(5):784-96. doi: 10.1111/acel.12356. Epub 2015 May 25. Aging Cell. 2015. PMID: 26010330 Free PMC article. - Neural stem cell- and neurogenesis-related gene expression profiles in the young and aged dentate gyrus.
Shetty GA, Hattiangady B, Shetty AK. Shetty GA, et al. Age (Dordr). 2013 Dec;35(6):2165-76. doi: 10.1007/s11357-012-9507-6. Epub 2013 Jan 16. Age (Dordr). 2013. PMID: 23322452 Free PMC article. - Fine processes of Nestin-GFP-positive radial glia-like stem cells in the adult dentate gyrus ensheathe local synapses and vasculature.
Moss J, Gebara E, Bushong EA, Sánchez-Pascual I, O'Laoi R, El M'Ghari I, Kocher-Braissant J, Ellisman MH, Toni N. Moss J, et al. Proc Natl Acad Sci U S A. 2016 May 3;113(18):E2536-45. doi: 10.1073/pnas.1514652113. Epub 2016 Apr 18. Proc Natl Acad Sci U S A. 2016. PMID: 27091993 Free PMC article. - New insights into mechanisms of stem cell daughter fate determination in regenerative tissues.
Sada A, Tumbar T. Sada A, et al. Int Rev Cell Mol Biol. 2013;300:1-50. doi: 10.1016/B978-0-12-405210-9.00001-1. Int Rev Cell Mol Biol. 2013. PMID: 23273858 Free PMC article. Review.
References
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. - PubMed
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. - PubMed
- Eckenhoff MMF, Rakic PP. Radial organization of the hippocampal dentate gyrus: a Golgi, ultrastructural, and immunocytochemical analysis in the developing rhesus monkey. Journal of comparative neurology. 1984;223:1–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases