Pericyte requirement for anti-leak action of angiopoietin-1 and vascular remodeling in sustained inflammation - PubMed (original) (raw)
. 2011 Jun;178(6):2897-909.
doi: 10.1016/j.ajpath.2011.02.008. Epub 2011 May 6.
Sébastien Tabruyn, Katharine Colton, Harras Zaid, Alicia Adams, Peter Baluk, Erin Lashnits, Tohru Morisada, Tom Le, Shaun O'Brien, David M Epstein, Gou Young Koh, Donald M McDonald
Affiliations
- PMID: 21550017
- PMCID: PMC3124300
- DOI: 10.1016/j.ajpath.2011.02.008
Pericyte requirement for anti-leak action of angiopoietin-1 and vascular remodeling in sustained inflammation
Jonas Fuxe et al. Am J Pathol. 2011 Jun.
Abstract
Blood vessel leakiness is an early, transient event in acute inflammation but can also persist as vessels undergo remodeling in sustained inflammation. Angiopoietin/Tie2 signaling can reduce the leakiness through changes in endothelial cells. The role of pericytes in this action has been unknown. We used the selective PDGF-B-blocking oligonucleotide aptamer AX102 to determine whether disruption of pericyte-endothelial crosstalk alters vascular leakiness or remodeling in the airways of mice under four different conditions: i) baseline, ii) acute inflammation induced by bradykinin, iii) sustained inflammation after 7-day infection by the respiratory pathogen Mycoplasma pulmonis, or iv) leakage after bradykinin challenge in the presence of vascular stabilization by the angiopoietin-1 (Ang1) mimic COMP-Ang1 for 7 days. AX102 reduced pericyte coverage but did not alter the leakage of microspheres from tracheal blood vessels at baseline or after bradykinin; however, AX102 exaggerated leakage at 7 days after M. pulmonis infection and increased vascular remodeling and disease severity at 14 days. AX102 also abolished the antileakage effect of COMP-Ang1 at 7 days. Together, these findings show that pericyte contributions to endothelial stability have greater dependence on PDGF-B during the development of sustained inflammation, when pericyte dynamics accompany vascular remodeling, than under baseline conditions or in acute inflammation. The findings also show that the antileakage action of Ang1 requires PDGF-dependent actions of pericytes in maintaining endothelial stability.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
Distribution of pericytes on vasculature of normal tracheal mucosa. A: Confocal microscopic image showing endothelial cells (green, PECAM-1) and pericytes (red, desmin) of the mucosal vasculature in a whole mount of normal mouse trachea. B–D: Higher magnification images with arrows marking desmin-positive smooth muscle cells in the wall of an arteriole (B) and pericytes in the wall of a capillary (C) and a venule (D). Scale bars: 100 μm (A); 20 μm (B–D).
Figure 2
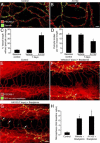
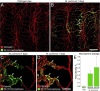
Effect of PDGF-B blockade by AX102 on pericyte coverage and bradykinin-induced leakage. A and B: Confocal microscopic images of the tracheal vasculature (endothelial cells, PECAM-1, red); compare the continuous row of pericytes (green, desmin) after treatment with vehicle (A) with incomplete pericyte coverage after AX102 for 7 days (arrow, B). C and D: Pericyte coverage of tracheal vasculature of baseline control mice versus mice treated with vehicle or AX102 for 7 days. The AX102 group had significantly less pericyte coverage (C) and fewer desmin-positive pericyte cell bodies (D). E–G: Fluorescence micrographs showing extravasated 500-nm microspheres (blue-white) at sites of leakage from vessels stained for PECAM-1 immunoreactivity (red). Bradykinin (1 mg/kg) was injected 1 minute before microspheres that circulated for 2 minutes before fixation by vascular perfusion. Microspheres are sparse in trachea of baseline control mouse after saline (E) but are abundant after bradykinin injected after 7-day treatment with vehicle (F). Leakage was not further increased by 7-day treatment with AX102 (G). Most extravasated microspheres are near postcapillary venules after vehicle (F, arrows), but after AX102 some microspheres extravasated from capillaries that cross cartilage rings (G, arrows). H: Abundance of extravasated microspheres (mean area density scaled to baseline control value = 1) in tracheas of the three groups. *P < 0.05 versus control. Scale bars: 20 μm (A and B); 50 μm (E–G).
Figure 3
Pericytes on tracheal vasculature after M. pulmonis infection. A–C: Confocal micrographs showing pericytes (red, desmin, arrows) and endothelial cells (PECAM-1, green) of normal capillaries (A) and remodeled vessels in tracheas at 7 days (B) or 14 days (C) after infection with M. pulmonis. Arrowheads in (B) mark desmin-positive cells not in contact with vasculature. Arrows in C mark abundance of pericytes on remodeled vessels after 14 days of infection. D and E: Higher magnification images; compare pericytes (desmin, black) on tracheal capillaries of pathogen-free mouse (D) with those on remodeled tracheal vessels after infection for 14 days (E). F–H: Number of pericyte cell bodies (F), percentage of vessel length with pericyte coverage (G), and PDGF-B mRNA expression normalized to β-actin × 10−3 (H) in tracheas of pathogen-free mice and mice infected for 7 or 14 days. *P < 0.05 versus pathogen-free; **P < 0.05 versus M. pulmonis infection for 7 days. Scale bars: 100 μm (A–C); 20 μm (D and E).
Figure 4
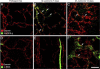
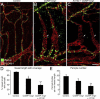
Pericyte changes after M. pulmonis infection. A–F: Confocal micrographs showing pericytes (desmin, red) on normal capillaries (A and D) and remodeled vessels in tracheas after M. pulmonis infection for 7 days (B and E) or 14 days (C and F). A–C: Pericytes with PDGFR-β immunoreactivity (green) are absent in normal capillaries (A) but are present after infection for 7 days (B, arrows). After 14 days of infection, round desmin-positive cells (C, arrows), some with faint PDGFR-β immunoreactivity, are scattered among blood vessels but do not resemble pericytes. D and E: Mural cells with α-SMA immunoreactivity (green) not present on capillaries of normal tracheas (D) or after 7 days of infection, except on arterioles (E), but are present on remodeled vessels (previously capillaries) after 14 days of infection (F, arrows). Scale bar = 50 μm (A–F).
Figure 5
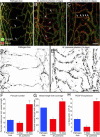
PDGF-B-dependent changes in pericytes after M. pulmonis infection. A–D: Confocal micrographs showing pericytes (desmin, red) and endothelial cells (PECAM-1, green) of remodeled tracheal blood vessels after M. pulmonis infection for 14 days with daily treatment with vehicle (A and B) or AX102 (C and D) during the final 7 days. The distribution of pericytes in A and C is highlighted in B and D, where desmin immunoreactivity is shown in black. After infection, pericytes are very numerous and densely packed (A and B), but with AX102 treatment the pericytes are more sparse, and pericyte-free regions are present on some vessels (C and D, arrows). E–G: Diameter of capillaries/remodeled vessels measured at the center of cartilage rings (E) and measurements by qPCR of expression of P-selectin mRNA (F) and of M. pulmonis 16S rRNA (G) in tracheas of pathogen-free mice or infected mice treated daily with vehicle or AX102 during the final 7 days of the 14-day infection. Expression values for P-selectin are normalized to β-actin × 10−3; values for M. pulmonis 16S rRNA are normalized to β-actin × 10. *P < 0.05 versus pathogen-free; **P < 0.05 versus vehicle treatment after infection. Scale bars = 15 μm (A–D).
Figure 6
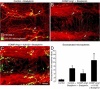
Microsphere leakage during M. pulmonis infection. A and B: Confocal microscopic images showing extravasated 50-nm microspheres (green) in the tracheal vasculature (PECAM-1, red) of a pathogen-free mouse (A) and a mouse infected with M. pulmonis for 7 days (B). C and D: Higher magnification images showing extravasated microspheres (green) in remodeled vessels with pericytes stained for desmin (C, red) or endothelial cells stained for PECAM-1 (D, red) after infection for 7 days. Arrows in C and D mark extravasated microspheres in pericyte-free regions of remodeled vessels. E: Abundance of extravasated microspheres, reflected by area density of microsphere fluorescence (threshold ≥50), in trachea of pathogen-free mice or mice infected with M. pulmonis for 7 days accompanied by treatment with vehicle or AX102. *P < 0.05 versus pathogen-free mice. Scale bars: 100 μm (A and B); 20 μm (C and D).
Figure 7
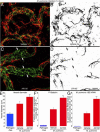
PDGF-dependent changes in pericytes after COMP-Ang1. A–C: Confocal micrographs of blood vessels (PECAM-1, red) in tracheal whole mounts showing a continuous, narrow row of pericytes (desmin, green) on capillaries in a control mouse treated with adenoviral LacZ (A) but pericyte-free regions of enlarged, remodeled vessels (arrows) in tracheas of mice treated with adenoviral COMP-Ang1 (B) or COMP-Ang1 with concurrent AX102 (C) for 7 days. Fewer pericytes are present on remodeled vessels in mice treated with AX102. D and E: Pericyte coverage, expressed as percentage of vessel length with pericytes (D) and number of pericyte cell bodies per millimeter (E), on tracheal vessels from pathogen-free mice treated for 7 days with adenoviral LacZ (control), COMP-Ang1, or COMP-Ang1 accompanied by AX102. *P < 0.05 versus vehicle; **P < 0.05 versus COMP-Ang1.
Figure 8
PDGF-B-dependence of antileakage effect of Ang1. A–C: Fluorescence micrographs of blood vessels (PECAM-1, red) in tracheal whole mounts from mice pretreated with adenoviral LacZ (control) (A) or COMP-Ang1 (B) or COMP-Ang1 accompanied by AX102 (C). After 7 days, all mice received an injection of bradykinin, followed 1 minute later by fluorescent 500-nm microspheres (blue-white, arrows), which circulated for 2 minutes before vascular perfusion of fixative. Many extravasated microspheres are visible in trachea prepared after bradykinin was preceded by adenoviral LacZ (A). Although few microspheres are visible when bradykinin was preceded by COMP-Ang1 (B), much leakage is evident when exposure to COMP-Ang1 was accompanied by PDGF-B blockade by daily doses of AX102 (C). D: Measurements of area density of extravasated microspheres in tracheas showed that AX102 treatment during COMP-Ang1 exposure increased the leakage effect of bradykinin by 61% over LacZ controls and by 162% over COMP-Ang1 without AX102. Values are scaled to the control (no bradykinin) group = 1. *P < 0.05 versus bradykinin group; **P < 0.05 versus COMP-Ang1 + bradykinin group. Scale bar = 30 μm (A–C).
Similar articles
- Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102.
Sennino B, Falcón BL, McCauley D, Le T, McCauley T, Kurz JC, Haskell A, Epstein DM, McDonald DM. Sennino B, et al. Cancer Res. 2007 Aug 1;67(15):7358-67. doi: 10.1158/0008-5472.CAN-07-0293. Cancer Res. 2007. PMID: 17671206 Free PMC article. - Synergistic actions of blocking angiopoietin-2 and tumor necrosis factor-α in suppressing remodeling of blood vessels and lymphatics in airway inflammation.
Le CT, Laidlaw G, Morehouse CA, Naiman B, Brohawn P, Mustelin T, Connor JR, McDonald DM. Le CT, et al. Am J Pathol. 2015 Nov;185(11):2949-68. doi: 10.1016/j.ajpath.2015.07.010. Epub 2015 Sep 5. Am J Pathol. 2015. PMID: 26348576 Free PMC article. - Angiopoietin-2-driven vascular remodeling in airway inflammation.
Tabruyn SP, Colton K, Morisada T, Fuxe J, Wiegand SJ, Thurston G, Coyle AJ, Connor J, McDonald DM. Tabruyn SP, et al. Am J Pathol. 2010 Dec;177(6):3233-43. doi: 10.2353/ajpath.2010.100059. Epub 2010 Oct 15. Am J Pathol. 2010. PMID: 20952594 Free PMC article. - Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy.
Ejaz S, Chekarova I, Ejaz A, Sohail A, Lim CW. Ejaz S, et al. Diabetes Obes Metab. 2008 Jan;10(1):53-63. doi: 10.1111/j.1463-1326.2007.00795.x. Epub 2007 Oct 15. Diabetes Obes Metab. 2008. PMID: 17941874 Review.
Cited by
- The angiopoietin-Tie2 signaling axis in the vascular leakage of systemic inflammation.
Milam KE, Parikh SM. Milam KE, et al. Tissue Barriers. 2015 Apr 3;3(1-2):e957508. doi: 10.4161/21688362.2014.957508. eCollection 2015. Tissue Barriers. 2015. PMID: 25838975 Free PMC article. - Dysfunctional resident lung mesenchymal stem cells contribute to pulmonary microvascular remodeling.
Chow K, Fessel JP, Kaoriihida-Stansbury, Schmidt EP, Gaskill C, Alvarez D, Graham B, Harrison DG, Wagner DH Jr, Nozik-Grayck E, West JD, Klemm DJ, Majka SM. Chow K, et al. Pulm Circ. 2013 Jan;3(1):31-49. doi: 10.4103/2045-8932.109912. Pulm Circ. 2013. PMID: 23662173 Free PMC article. - Tumor stroma as targets for cancer therapy.
Zhang J, Liu J. Zhang J, et al. Pharmacol Ther. 2013 Feb;137(2):200-15. doi: 10.1016/j.pharmthera.2012.10.003. Epub 2012 Oct 12. Pharmacol Ther. 2013. PMID: 23064233 Free PMC article. Review. - Angiopoietin-Tie signalling in the cardiovascular and lymphatic systems.
Eklund L, Kangas J, Saharinen P. Eklund L, et al. Clin Sci (Lond). 2017 Jan 1;131(1):87-103. doi: 10.1042/CS20160129. Clin Sci (Lond). 2017. PMID: 27941161 Free PMC article. Review. - VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling.
Heinolainen K, Karaman S, D'Amico G, Tammela T, Sormunen R, Eklund L, Alitalo K, Zarkada G. Heinolainen K, et al. Circ Res. 2017 Apr 28;120(9):1414-1425. doi: 10.1161/CIRCRESAHA.116.310477. Epub 2017 Mar 15. Circ Res. 2017. PMID: 28298294 Free PMC article.
References
- Armulik A., Abramsson A., Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. - PubMed
- Gaengel K., Genove G., Armulik A., Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. - PubMed
- von Tell D., Armulik A., Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–629. - PubMed
- Speyer C.L., Steffes C.P., Ram J.L. Effects of vasoactive mediators on the rat lung pericyte: quantitative analysis of contraction on collagen lattice matrices. Microvasc Res. 1999;57:134–143. - PubMed
- Sims D.E. Diversity within pericytes. Clin Exp Pharmacol Physiol. 2000;27:842–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL-24136/HL/NHLBI NIH HHS/United States
- P01 HL024136/HL/NHLBI NIH HHS/United States
- R01 HL059157/HL/NHLBI NIH HHS/United States
- R01 HL096511/HL/NHLBI NIH HHS/United States
- HL-59157/HL/NHLBI NIH HHS/United States
- HL-096511/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous