Complex interactions between genes controlling trafficking in primary cilia - PubMed (original) (raw)
Complex interactions between genes controlling trafficking in primary cilia
Polloneal Jymmiel R Ocbina et al. Nat Genet. 2011 Jun.
Abstract
Cilia-associated human genetic disorders are striking in the diversity of their abnormalities and their complex inheritance. Inactivation of the retrograde ciliary motor by mutations in DYNC2H1 causes skeletal dysplasias that have strongly variable expressivity. Here we define previously unknown genetic relationships between Dync2h1 and other genes required for ciliary trafficking. Mutations in mouse Dync2h1 disrupt cilia structure, block Sonic hedgehog signaling and cause midgestation lethality. Heterozygosity for Ift172, a gene required for anterograde ciliary trafficking, suppresses cilia phenotypes, Sonic hedgehog signaling defects and early lethality of Dync2h1 homozygotes. Ift122, like Dync2h1, is required for retrograde ciliary trafficking, but reduction of Ift122 gene dosage also suppresses the Dync2h1 phenotype. These genetic interactions illustrate the cell biology underlying ciliopathies and argue that mutations in intraflagellar transport genes cause their phenotypes because of their roles in cilia architecture rather than direct roles in signaling.
Figures
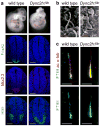
Figure 1. Mutations in Dync2h1 disrupt Shh-dependent neural patterning and cilia morphology
(a), Mutations in Dync2h1 lead to the absence of Shh-dependent cell types in the E10.5 neural tube. In Dync2h1lln/lln mutants, floor plate (FoxA2, green) and V3 progenitor (Nkx2.2, red) domains are not specified, and motor neurons (HB9, green) are present only in the caudal neural tube (shown here); dorsal up. Scale bars represent 100 μm. (b), Scanning electron micrographs show that neural tube primary cilia in Dync2h1lln/lln mutants are bloated; dimensions are given in Supplementary Table1. Scale bars represent 500 nm. (c), IFT88 (green) in cilia of serum-starved wild-type MEFs is enriched at the base and the tip of the cilium, marked with acetylated α-tubulin (red). In Dync2h1lln/lln mutant MEFs, the amount of IFT88 in the cilium is increased and is found all along the axoneme. Quantitation is in Supplementary Table 2. Scale bars represent 1 μm (c).
Figure 2. Hh components accumulate in Dync2h1 mutant cilia
Localization of Smo (a, b), Gli2 (c, d) and Ptch1 (e, f) to the primary cilium in wild-type and Dync2h1lln/lln MEFs (a, c, e) and E10.5 neural tube (b, d, f). (a) Smo (green) was enriched in cilia of wild-type MEFs only after exposure to Shh. Smo was enriched in cilia of Dync2h1lln/lln mutant cells even in the absence of Shh. (b) Smo was enriched in cilia of ventral neural progenitors in wild-type. Smo was strongly enriched in primary cilia of Dync2h1lln/lln neural progenitors at all dorsal-ventral levels. (c) Gli2 (green) localized to the tips of cilia in wild-type MEFs and accumulated further after Shh treatment. Gli2 levels were elevated along the axoneme of Dync2h1lln/lln mutant MEF cilia. (d) Gli2 was elevated in the cilia of Dync2h1lln/lln neural progenitors. (e) Low amounts of endogenous Ptch1 (green) were detected near the base and along the length of primary cilia in wild-type MEFs only in the absence of Shh, whereas Ptch1 was strongly enriched along the axoneme of Dync2h1lln/lln cilia in unstimulated cells; strong Ptch1 immunofluorescence remained near the base of the cilium after stimulation with Shh. (f) Ptch1 appeared localized to the cytoplasm of wild-type neural progenitors, and was strongly enriched in cilia throughout the neural tube in Dync2h1lln/lln mutants. Acetylated α-tubulin (red) marks cilia in (a, c, e); (b, d, f) are ventral views of transverse sections through the ventral half of the neural tube at the level of the forelimb. Scale bars represent 500 nm (a, c, e), 25 μm (b, d, f) and 10 μm (insets b, d, f).
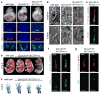
Figure 3. Ift172 is a dominant suppressor of Dync2h1
(a), E10.5 embryos and transverse sections through the caudal neural tube of wild-type, Dync2h1lln/lln and Dync2h1lln/lln Ift172avc1/+ embryos. Specification of floor plate (FoxA2, green), V3 progenitors (Nkx2.2, red) and motor neurons (HB9, green) were rescued in Dync2h1lln/lln Ift172avc1/+ embryos. Scale bars represent 100 μm. (b), Dync2h1lln/lln Ift172avc1/+ mutants survive to at least E16.5 (n=5). Scale bar is 5 mm. (c), Right forelimbs and digits of embryos in (b) stained with Alcian blue (cartilage) and Alizarin red (bone) staining shows incomplete penetrance of polydactyly in Dync2h1lln/lln Ift172avc1/+ embryos at E16.5. (d), Scanning electron micrographs of cilia from the neural tube at E10.5 showing near-normal morphology of Dync2h1lln/lln Ift172avc1/+ mutant cilia (quantitation in Supplementary Table 1). Scale bar is 500 nm. IFT88 (green, e) and Smo (green, f) and Gli2 (red, g) localize normally in primary cilia of MEFs derived from Dync2h1lln/lln Ift172avc1/+ embryos. Acetylated α-tubulin (red) marks cilia in (e-g). Scale bars represent 1 μm in (e-g).
Figure 4. Neural patterning and cilia morphology in Dync2h1lln Ift122sopb embryos
(a), In contrast to the lack of ventral neural cell types in Dync2h1lln/lln mutants, both Ift122sopb/sopb single and Dync2h1lln/lln Ift122sopb/sopb double mutants specify floor plate (FoxA2, green), V3 progenitors (Nkx2.2, red) and motor neurons (HB9, green) in the lumbar neural tube. Scale bars represent 100 μm. (b), Scanning electron micrographs of neural tube cilia from the neural tube of E10.5 Ift122sopb/sopb and Dync2h1lln/lln Ift122sopb/sopb embryos. The distal ends of Ift122sopb/sopb mutant cilia appeared swollen. Dync2h1lln/lln Ift122sopb/sopb mutant cilia were similar in diameter to Ift122sopb/sopb but were shorter than either Dync2h1lln/lln or Ift122sopb/sopb single mutants (See Supplementary Table 1). Scale bars represent 500 nm. (c), IFT88 (green) accumulates specifically at the distal tips of both Ift122sopb/sopb and Dync2h1lln/lln Ift122sopb/sopb mutant MEF cilia. Acetylated α-tubulin staining (red) marks primary cilia. Localization of Smo (d, green) and Gli2 (e, green) in the cilia of Ift122sopb/sopb and Dync2h1lln/lln Ift122sopb/sopb mutant MEFs. Acetylated α-tubulin (red) marks cilia. (f), Dync2h1 protein is present at the base of the cilium and along the ciliary axoneme in wild-type cells. In Ift122sopb/sopb mutant cilia, Dync2h1 localization accumulates mainly at the base of the cilium. Orientation for (b-f) is distal tip up. Scale bars are 1 μm (d-f).
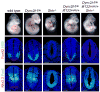
Figure 5. Shh responsiveness in Dync2h1lln/lln Ift122sopb/+ embryos
Whole embryos and patterning in the lumbar neural tube of E10.5 wild type, Dync2h1lln/lln, Shh-/-, Dync2h1lln/lln Ift122sopb/+ and Dync2h1lln/lln Ift122sopb/+ Shh-/- compound mutants. Specification of floor plate (FoxA2, red, middle panels), V3 progenitors (Nkx2.2, red, bottom panels) and motor neurons (HB9, green, middle panels) were partially rescued in Dync2h1lln/lln Ift122sopb/+ mutant embryos but all these cell types were absent in Dync2h1lln/lln Ift122sopb/+ Shh-/- embryos. The Pax6 domain (green, bottom panels), which is restricted by low levels of Shh signaling, was ventrally expanded in Shh-/- and Dync2h1lln/lln Ift122sopb/+ Shh-/- embryos. Scale bars represent 100 μm.
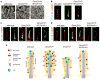
Figure 6. Cilia morphology in Dync2h1lln/lln Ift122sopb/+ compound mutants
(a), SEM analysis of neural tube primary cilia show the more normal length and width of Dync2h1lln/lln Ift122sopb/+ mutants compared to Dync2h1lln/lln. Quantitation in Supplementary Table 1. Scale bars are 500 nm. (b-d), Localization of IFT88 (b, green), Smo (c, green) and Gli2 (d, green) in cilia (acetylated α-tubulin, red) appear normal in Dync2h1lln/lln Ift122sopb/+ mutants. Scale bars are 500 nm (b-d). (e) Model of the trafficking of mammalian IFT and Hh pathway proteins in the primary cilium, shown in the absence of Hh ligand. In wild-type cells, IFT directs the formation of cilia, which accumulate a basal level of Gli2 at cilia tips, while Smo traffics through the cilium at a low basal rate. Loss of retrograde motor in Dync2h1lln/lln mutant cilia leads to the accumulation of IFT particles and blocks the movement of both Smo and Gli2 out of the cilium. In Ift122sopb/sopb mutants, Dync2h1 protein fails to enter the cilium, leading to the accumulation of IFT-B particles. Loss of IFT122 also results in the accumulation of Gli2 but does not affect Smo trafficking. Decreased anterograde ciliary trafficking in Dync2h1lln/lln Ift122sopb/+ suppresses the Dync2h1lln/lln phenotype and permits normal transport of both Smo and Gli2 through the cilium.
Similar articles
- Loss of dynein-2 intermediate chain Wdr34 results in defects in retrograde ciliary protein trafficking and Hedgehog signaling in the mouse.
Wu C, Li J, Peterson A, Tao K, Wang B. Wu C, et al. Hum Mol Genet. 2017 Jul 1;26(13):2386-2397. doi: 10.1093/hmg/ddx127. Hum Mol Genet. 2017. PMID: 28379358 Free PMC article. - Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components.
Qin J, Lin Y, Norman RX, Ko HW, Eggenschwiler JT. Qin J, et al. Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1456-61. doi: 10.1073/pnas.1011410108. Epub 2011 Jan 5. Proc Natl Acad Sci U S A. 2011. PMID: 21209331 Free PMC article. - Combinations of deletion and missense variations of the dynein-2 DYNC2LI1 subunit found in skeletal ciliopathies cause ciliary defects.
Qiu H, Tsurumi Y, Katoh Y, Nakayama K. Qiu H, et al. Sci Rep. 2022 Jan 7;12(1):31. doi: 10.1038/s41598-021-03950-0. Sci Rep. 2022. PMID: 34997029 Free PMC article. - Architecture of the IFT ciliary trafficking machinery and interplay between its components.
Nakayama K, Katoh Y. Nakayama K, et al. Crit Rev Biochem Mol Biol. 2020 Apr;55(2):179-196. doi: 10.1080/10409238.2020.1768206. Epub 2020 May 26. Crit Rev Biochem Mol Biol. 2020. PMID: 32456460 Review. - Coordinating the uncoordinated: UNC119 trafficking in cilia.
Jean F, Pilgrim D. Jean F, et al. Eur J Cell Biol. 2017 Oct;96(7):643-652. doi: 10.1016/j.ejcb.2017.09.001. Epub 2017 Sep 6. Eur J Cell Biol. 2017. PMID: 28935136 Review.
Cited by
- Genome-wide copy number analysis uncovers a new HSCR gene: NRG3.
Tang CS, Cheng G, So MT, Yip BH, Miao XP, Wong EH, Ngan ES, Lui VC, Song YQ, Chan D, Cheung K, Yuan ZW, Lei L, Chung PH, Liu XL, Wong KK, Marshall CR, Scherer SW, Cherny SS, Sham PC, Tam PK, Garcia-Barceló MM. Tang CS, et al. PLoS Genet. 2012;8(5):e1002687. doi: 10.1371/journal.pgen.1002687. Epub 2012 May 10. PLoS Genet. 2012. PMID: 22589734 Free PMC article. - TCTEX1D2 mutations underlie Jeune asphyxiating thoracic dystrophy with impaired retrograde intraflagellar transport.
Schmidts M, Hou Y, Cortés CR, Mans DA, Huber C, Boldt K, Patel M, van Reeuwijk J, Plaza JM, van Beersum SE, Yap ZM, Letteboer SJ, Taylor SP, Herridge W, Johnson CA, Scambler PJ, Ueffing M, Kayserili H, Krakow D, King SM; UK10K; Beales PL, Al-Gazali L, Wicking C, Cormier-Daire V, Roepman R, Mitchison HM, Witman GB. Schmidts M, et al. Nat Commun. 2015 Jun 5;6:7074. doi: 10.1038/ncomms8074. Nat Commun. 2015. PMID: 26044572 Free PMC article. - Nonredundant roles of DIAPHs in primary ciliogenesis.
Palander O, Lam A, Collins RF, Moraes TJ, Trimble WS. Palander O, et al. J Biol Chem. 2021 Jan-Jun;296:100680. doi: 10.1016/j.jbc.2021.100680. Epub 2021 Apr 17. J Biol Chem. 2021. PMID: 33872598 Free PMC article. - LRRK1-mediated NDEL1 phosphorylation promotes cilia disassembly via dynein-2-driven retrograde intraflagellar transport.
Hanafusa H, Kedashiro S, Gotoh M, Saitoh KH, Inaba H, Nishioka T, Kaibuchi K, Inagaki M, Hisamoto N, Matsumoto K. Hanafusa H, et al. J Cell Sci. 2022 Nov 1;135(21):jcs259999. doi: 10.1242/jcs.259999. Epub 2022 Nov 4. J Cell Sci. 2022. PMID: 36254578 Free PMC article. - Ciliary exclusion of Polycystin-2 promotes kidney cystogenesis in an autosomal dominant polycystic kidney disease model.
Walker RV, Keynton JL, Grimes DT, Sreekumar V, Williams DJ, Esapa C, Wu D, Knight MM, Norris DP. Walker RV, et al. Nat Commun. 2019 Sep 6;10(1):4072. doi: 10.1038/s41467-019-12067-y. Nat Commun. 2019. PMID: 31492868 Free PMC article.
References
- Badano JL, et al. Dissection of epistasis in oligogenic Bardet-Biedl syndrome. Nature. 2006;439:326–30. - PubMed
- Hoefele J, et al. Evidence of oligogenic inheritance in nephronophthisis. J Am Soc Nephrol. 2007;18:2789–95. - PubMed
- Katsanis N, et al. Triallelic inheritance in Bardet-Biedl syndrome, a Mendelian recessive disorder. Science. 2001;293:2256–9. - PubMed
- Leitch CC, et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet. 2008;40:443–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous