Neuroprotection mediated through estrogen receptor-alpha in astrocytes - PubMed (original) (raw)
. 2011 May 24;108(21):8867-72.
doi: 10.1073/pnas.1103833108. Epub 2011 May 9.
Mary E Hamby, Elizabeth Umeda, Noriko Itoh, Sienmi Du, Amy J Wisdom, Yuan Cao, Galyna Bondar, Jeannie Lam, Yan Ao, Francisco Sandoval, Silvie Suriany, Michael V Sofroniew, Rhonda R Voskuhl
Affiliations
- PMID: 21555578
- PMCID: PMC3102368
- DOI: 10.1073/pnas.1103833108
Neuroprotection mediated through estrogen receptor-alpha in astrocytes
Rory D Spence et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Estrogen has well-documented neuroprotective effects in a variety of clinical and experimental disorders of the CNS, including autoimmune inflammation, traumatic injury, stroke, and neurodegenerative diseases. The beneficial effects of estrogens in CNS disorders include mitigation of clinical symptoms, as well as attenuation of histopathological signs of neurodegeneration and inflammation. The cellular mechanisms that underlie these CNS effects of estrogens are uncertain, because a number of different cell types express estrogen receptors in the peripheral immune system and the CNS. Here, we investigated the potential roles of two endogenous CNS cell types in estrogen-mediated neuroprotection. We selectively deleted estrogen receptor-α (ERα) from either neurons or astrocytes using well-characterized Cre-loxP systems for conditional gene knockout in mice, and studied the effects of these conditional gene deletions on ERα ligand-mediated neuroprotective effects in a well-characterized model of adoptive experimental autoimmune encephalomyelitis (EAE). We found that the pronounced and significant neuroprotective effects of systemic treatment with ERα ligand on clinical function, CNS inflammation, and axonal loss during EAE were completely prevented by conditional deletion of ERα from astrocytes, whereas conditional deletion of ERα from neurons had no significant effect. These findings show that signaling through ERα in astrocytes, but not through ERα in neurons, is essential for the beneficial effects of ERα ligand in EAE. Our findings reveal a unique cellular mechanism for estrogen-mediated CNS neuroprotective effects by signaling through astrocytes, and have implications for understanding the pathophysiology of sex hormone effects in diverse CNS disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
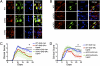
Fig. 1.
(A and B) Verification of gene deletion specificity in astrocyte-ERα-CKO (aCKO) and neuronal-ERα-CKO (nCKO) mouse models. (C and D) EAE clinical disease severity scores showing that protective effects of ERα ligand require ERα in astrocytes, but not neurons. (A) Immunohistochemistry shows ERα colocalized with NeuN and DAPI in WT and aCKO mice with EAE, but not in nCKO mice with EAE. (B) ERα is colocalized with GFAP and DAPI in WT and nCKO mice with EAE, but not in aCKO mice with EAE. (Scale bars, 15 μm.) (C) WT and nCKO mice with EAE and given ERα ligand both had significantly better clinical scores compared with WT and nCKO mice with EAE and given vehicle. n = 6 per group. (D) Only WT mice, but not aCKO mice, with EAE and given ERα ligand had significantly better clinical scores compared with WT or aCKO mice with EAE and given vehicle. n = 12 per group. *P < 0.05 (repeated-measures ANOVA with post hoc Bonferroni pairwise analysis).
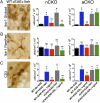
Fig. 2.
Immunohistochemical evidence that ERα is required in astrocytes, but not neurons, to reduce numbers of Iba-1 globoid macrophages and CD3 T cells in dorsal column white matter. (A) Iba-1 globoid macrophages were significantly reduced in WT and nCKO mice with EAE treated with ERα ligand, but not in aCKO mice with EAE treated with ERα ligand. (B) Iba-1 ramified microglia exhibited no significant difference in number across all experimental groups. (C) CD3 T cells were reduced in WT and nCKO mice with EAE treated with ERα ligand, but not in aCKO mice with EAE treated with ERα ligand. (Scale bar, 15 μm.) n = 6 per group. *P < 0.05; NS, not significant vs. WT+No EAE; #P < 0.05 vs. WT+EAE+Veh, aCKO+EAE+Veh, or aCKO+EAE+ERα ligand (ANOVA with post hoc Bonferroni pairwise analysis).
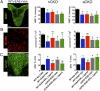
Fig. 3.
Flow cytometry evidence that ERα is required in astrocytes, but not neurons, to reduce macrophage and T-cell inflammation. (A and C) Macrophages (R1) (CD11bhi/CD45hi) and (B and C) T-cells (CD45hi/CD3hi) were significantly reduced in WT, but not aCKO mice, treated with ERα ligand via flow cytometry from the CNS. (A and C) There were no significant differences among all groups in numbers of microglia (R2) (CD11bhi/CD45int). n = 5 per group. *P < 0.05. NS, not significant vs. WT+EAE+ERα ligand (ANOVA with post hoc Bonferroni pairwise analysis).
Fig. 4.
Immunohistochemical evidence that ERα is required in astrocytes, but not neurons, to protect against axonal loss and reactive astrogliosis. (A) MBP stained intensity and area were significantly reduced in all EAE groups, but were not significantly altered by ERα ligand treatment in any group. (Scale bar, 120 μm.) (B) Numbers of NF200+ axons were significantly reduced in WT mice with EAE; and treatment with ERα ligand ameliorated axonal loss in WT and nCKO mice, but not aCKO mice, with EAE. (Scale bar, 20 μm.) (C) GFAP stained area was significantly increased in WT mice with EAE; and treatment with ERα ligand ameliorated this increase in WT and nCKO mice, but not aCKO mice, with EAE. (Scale bar, 40 μm.) n = 6 per group. *P < 0.05; NS, not significant vs. WT+No EAE (ANOVA with post hoc Bonferroni pairwise analysis).
Similar articles
- Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERα signaling on astrocytes but not through ERβ signaling on astrocytes or neurons.
Spence RD, Wisdom AJ, Cao Y, Hill HM, Mongerson CR, Stapornkul B, Itoh N, Sofroniew MV, Voskuhl RR. Spence RD, et al. J Neurosci. 2013 Jun 26;33(26):10924-33. doi: 10.1523/JNEUROSCI.0886-13.2013. J Neurosci. 2013. PMID: 23804112 Free PMC article. - Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis.
Morales LB, Loo KK, Liu HB, Peterson C, Tiwari-Woodruff S, Voskuhl RR. Morales LB, et al. J Neurosci. 2006 Jun 21;26(25):6823-33. doi: 10.1523/JNEUROSCI.0453-06.2006. J Neurosci. 2006. PMID: 16793889 Free PMC article. - Neuroprotective and anti-inflammatory effects of estrogen receptor ligand treatment in mice.
Tiwari-Woodruff S, Voskuhl RR. Tiwari-Woodruff S, et al. J Neurol Sci. 2009 Nov 15;286(1-2):81-5. doi: 10.1016/j.jns.2009.04.023. Epub 2009 May 13. J Neurol Sci. 2009. PMID: 19442988 Free PMC article. - Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration.
Spence RD, Voskuhl RR. Spence RD, et al. Front Neuroendocrinol. 2012 Jan;33(1):105-15. doi: 10.1016/j.yfrne.2011.12.001. Epub 2011 Dec 24. Front Neuroendocrinol. 2012. PMID: 22209870 Free PMC article. Review. - Estrogen receptor agonists for attenuation of neuroinflammation and neurodegeneration.
Chakrabarti M, Haque A, Banik NL, Nagarkatti P, Nagarkatti M, Ray SK. Chakrabarti M, et al. Brain Res Bull. 2014 Oct;109:22-31. doi: 10.1016/j.brainresbull.2014.09.004. Epub 2014 Sep 20. Brain Res Bull. 2014. PMID: 25245209 Free PMC article. Review.
Cited by
- Sex Differences in Astrocyte Activity.
Gozlan E, Lewit-Cohen Y, Frenkel D. Gozlan E, et al. Cells. 2024 Oct 18;13(20):1724. doi: 10.3390/cells13201724. Cells. 2024. PMID: 39451242 Free PMC article. Review. - Underlying Mechanism of Lysosomal Membrane Permeabilization in CNS Injury: A Literature Review.
Xiang L, Lou J, Zhao J, Geng Y, Zhang J, Wu Y, Zhao Y, Tao Z, Li Y, Qi J, Chen J, Yang L, Zhou K. Xiang L, et al. Mol Neurobiol. 2024 Jun 18. doi: 10.1007/s12035-024-04290-6. Online ahead of print. Mol Neurobiol. 2024. PMID: 38888836 Review. - Ultrasound and neuroinflammation: immune modulation via the heat shock response.
Seasons GM, Pellow C, Kuipers HF, Pike GB. Seasons GM, et al. Theranostics. 2024 May 19;14(8):3150-3177. doi: 10.7150/thno.96270. eCollection 2024. Theranostics. 2024. PMID: 38855178 Free PMC article. Review. - Sex differences in the neuronal transcriptome and synaptic mitochondrial function in the cerebral cortex of a multiple sclerosis model.
Itoh N, Itoh Y, Stiles L, Voskuhl R. Itoh N, et al. Front Neurol. 2023 Nov 2;14:1268411. doi: 10.3389/fneur.2023.1268411. eCollection 2023. Front Neurol. 2023. PMID: 38020654 Free PMC article. - Relationship Between a High-Fat Diet, Reduced Mobility, and Trace Element Overload in the Olfactory Bulbs of C57BL/6J and DBA/2J Mice.
Totten MS, Howell JM, Tomberlin JA, Erikson KM. Totten MS, et al. Biol Trace Elem Res. 2024 Jul;202(7):3215-3224. doi: 10.1007/s12011-023-03911-w. Epub 2023 Oct 21. Biol Trace Elem Res. 2024. PMID: 37864044
References
- Lee SJ, McEwen BS. Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annu Rev Pharmacol Toxicol. 2001;41:569–591. - PubMed
- Wise PM. Estrogens and neuroprotection. Trends Endocrinol Metab. 2002;13:229–230. - PubMed
- Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR. Estriol ameliorates autoimmune demyelinating disease: Implications for multiple sclerosis. Neurology. 1999;52:1230–1238. - PubMed
- Trapp BD, Nave K-A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HD007228/HD/NICHD NIH HHS/United States
- T32 GM065823/GM/NIGMS NIH HHS/United States
- R01 NS057624/NS/NINDS NIH HHS/United States
- K24 NS062117/NS/NINDS NIH HHS/United States
- NS 062117/NS/NINDS NIH HHS/United States
- NS057624/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases