Neuronal life span versus health span: principles of natural selection at work in the degenerating brain - PubMed (original) (raw)
Review
Neuronal life span versus health span: principles of natural selection at work in the degenerating brain
John C O'Leary 3rd et al. J Mol Neurosci. 2011 Nov.
Abstract
Impaired nutrient delivery to the brain due to decreased blood flow contributes to cognitive decline and dementia in Alzheimer's disease (AD). Considering this, many studies have suggested that neuroprotective agents like those used in stroke could prevent AD onset or progression by promoting cell survival. However, research in the past decade suggests that the culprit behind the cognitive loss in AD models is actually the soluble tau accumulating inside of surviving neurons. In fact, tau reductions improve cognition in mouse models of AD, even those that only deposit amyloid plaques. There is emerging evidence that neuroprotection alone in these AD models may be insufficient to restore neuron function and cognition. Only when soluble tau is reduced on a neuroprotective background could memory be rescued. Thus, once a neuron begins to accumulate tau, it may survive in a malfunctioning capacity, leading to impaired electrical signaling and memory formation in the brain. These data imply that multiple drugs may be necessary to ameliorate the different disease components. In fact, strategies to preserve neurons without affecting the soluble protein burden within neurons may accelerate the disease course.
Figures
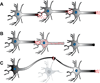
Figure 1
Sub-optimally functioning neurons surviving with proteotoxic tau accumulation subvert brain plasticity that normally occurs in response to neuronal death. (A) Neuronal transmission in normal brain. (B) Sub-optimally performing neurons due to tau accumulation cannot transmit a signal to the post-synaptic neuron, and the brain fails to re-route connectivity since the neuron is not dead. (C) Neuronal death facilitates network re-routing, allowing the plastic brain to adapt and re-establish downstream connectivity.
Similar articles
- Role of natural products for the treatment of Alzheimer's disease.
Noori T, Dehpour AR, Sureda A, Sobarzo-Sanchez E, Shirooie S. Noori T, et al. Eur J Pharmacol. 2021 May 5;898:173974. doi: 10.1016/j.ejphar.2021.173974. Epub 2021 Feb 27. Eur J Pharmacol. 2021. PMID: 33652057 Review. - In vivo modification of Abeta plaque toxicity as a novel neuroprotective lithium-mediated therapy for Alzheimer's disease pathology.
Trujillo-Estrada L, Jimenez S, De Castro V, Torres M, Baglietto-Vargas D, Moreno-Gonzalez I, Navarro V, Sanchez-Varo R, Sanchez-Mejias E, Davila JC, Vizuete M, Gutierrez A, Vitorica J. Trujillo-Estrada L, et al. Acta Neuropathol Commun. 2013 Nov 12;1:73. doi: 10.1186/2051-5960-1-73. Acta Neuropathol Commun. 2013. PMID: 24252759 Free PMC article. - Ebselen ameliorates β-amyloid pathology, tau pathology, and cognitive impairment in triple-transgenic Alzheimer's disease mice.
Xie Y, Tan Y, Zheng Y, Du X, Liu Q. Xie Y, et al. J Biol Inorg Chem. 2017 Aug;22(6):851-865. doi: 10.1007/s00775-017-1463-2. Epub 2017 May 13. J Biol Inorg Chem. 2017. PMID: 28502066 - Neuroprotective Studies of Evodiamine in an Okadaic Acid-Induced Neurotoxicity.
Chou CH, Yang CR. Chou CH, et al. Int J Mol Sci. 2021 May 19;22(10):5347. doi: 10.3390/ijms22105347. Int J Mol Sci. 2021. PMID: 34069531 Free PMC article. - Alzheimer's disease and amyloid: culprit or coincidence?
Skaper SD. Skaper SD. Int Rev Neurobiol. 2012;102:277-316. doi: 10.1016/B978-0-12-386986-9.00011-9. Int Rev Neurobiol. 2012. PMID: 22748834 Review.
Cited by
- Accelerated neurodegeneration through chaperone-mediated oligomerization of tau.
Blair LJ, Nordhues BA, Hill SE, Scaglione KM, O'Leary JC 3rd, Fontaine SN, Breydo L, Zhang B, Li P, Wang L, Cotman C, Paulson HL, Muschol M, Uversky VN, Klengel T, Binder EB, Kayed R, Golde TE, Berchtold N, Dickey CA. Blair LJ, et al. J Clin Invest. 2013 Oct;123(10):4158-69. doi: 10.1172/JCI69003. Epub 2013 Sep 3. J Clin Invest. 2013. PMID: 23999428 Free PMC article.
References
- Albani D, Polito L, Signorini A, Forloni G. Neuroprotective properties of resveratrol in different neurodegenerative disorders. Biofactors. 2010;36:370–376. - PubMed
- Andorfer C, Kress Y, Espinoza M, et al. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous