An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation - PubMed (original) (raw)
An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation
Kaoru Saijo et al. Cell. 2011.
Abstract
Microglia and astrocytes play essential roles in the maintenance of homeostasis within the central nervous system, but mechanisms that control the magnitude and duration of responses to infection and injury remain poorly understood. Here, we provide evidence that 5-androsten-3β,17β-diol (ADIOL) functions as a selective modulator of estrogen receptor (ER)β to suppress inflammatory responses of microglia and astrocytes. ADIOL and a subset of synthetic ERβ-specific ligands, but not 17β-estradiol, mediate recruitment of CtBP corepressor complexes to AP-1-dependent promoters, thereby repressing genes that amplify inflammatory responses and activate Th17 T cells. Reduction of ADIOL or ERβ expression results in exaggerated inflammatory responses to TLR4 agonists. Conversely, the administration of ADIOL or synthetic ERβ-specific ligands that promote CtBP recruitment prevents experimental autoimmune encephalomyelitis in an ERβ-dependent manner. These findings provide evidence for an ADIOL/ERβ/CtBP-transrepression pathway that regulates inflammatory responses in microglia and can be targeted by selective ERβ modulators.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
None
Figures
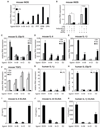
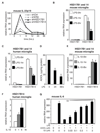
Figure 1. Indazole-estrogens repress inflammatory responses in microglia
A. Indazole-Br and Indazole-Cl inhibit LPS induction of iNOS mRNA 6 hr after LPS stimulation of murine BV2 microglia cells. *p < 0.01 compared to EtOH sample. B. Inhibitory effects of Indazole-Cl on LPS induction of iNOS in BV2 cells are abolished by knockdown of ERβ expression. *p < 0.01 compared to control siRNA transfected samples. C–F. Effects of Indazole-Br and Indazole-Cl on LPS induction of IL-23p19 (C), IL-6 (D), IL-1β (E) and _TGF_β (F) mRNAs in BV2 cells. *p < 0.01 compared to EtOH treated 1hr LPS stimulated sample, **p < 0.01 compared to EtOH treated 6hr LPS stimulated sample. G–H. Indazole-Br and Indazole-Cl inhibit LPS induction of _IL-1_β (G) and IL-23p19 (H) mRNAs (black bar) in human primary microglia cells. *p < 0.01 compared to 1 hr LPS + EtOH-stimulated sample, *p < 0.01 compared to 6 hr LPS + EtOH-stimulated sample. I–K. Indazole-Br and Indazole-Cl inhibit LPS-induced secretion of mouse IL-6 (I), mouse IL-23 (J) and human IL-1β 24hr after stimulation of corresponding primary mouse and human microglia cells as determined by ELISA. *p < 0.01 compared to EtOH treated sample. Error bars represent SD. In-Br and In-Cl are Indazole-Br and Indazole-Cl, respectively. E2 is 17β-estradiol. See also Figure S1.
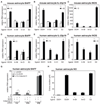
Figure 2. Indazole-estrogens repress inflammatory responses of astrocytes
A–C. Indazole-Br and Indazole-Cl inhibit IL-1β-dependent induction of BAFF (A), IL-23p19 (B) and iNOS (C) mRNAs in primary mouse astrocytes. *p < 0.01 compared to EtOH treated IL1β stimulated sample, **p < 0.01 compared to EtOH treated 6 hr LPS stimulated sample. D–F. Indazole-Br and Indazole-Cl inhibit IL-1β-dependent induction of BAFF (D), IL-23p19 (E) and iNOS (F) mRNAs in primary human astrocytes. **p < 0.01 compared to EtOH treated 6 hr IL-1β stimulated sample. # p < 0.01 compared to EtOH treated non-stimulated sample. G. siRNA-mediated knockdown of ERβ in astrocytes results in exaggerated BAFF expression in response to IL1β and abolishes the inhibitory effects of Indazole-Cl. H. Indazole-Br and Indazole-Cl inhibit IL1β-dependent production of nitric oxide (NO) by human primary astrocytes as determined by the Greiss reaction. *p < 0.01 compared to EtOH treated and IL-1β stimulated sample. Error bars represent SD. See also Figure S2.
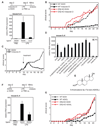
Figure 3. Indazole-Cl and ADIOL exert anti-inflammatory effects in vivo and inhibit EAE dependent on ERβ
A. Systemic administration of Indazole-Cl (approximately 2.4 µmol/kg/day) blocks the ability of LPS injection (10 mg/kg) to induce IL-6 expression in the substantia nigra (n=4/group). Data are representative of three independent experiments. B. Indazole-Cl (2.4 µmol/kg/day) inhibits development of EAE in wild-type female mice, but not in ERβ knockout female mice. Clinical scores are indicated for age matched wild-type mice treated with EtOH (n=24, black dashed line), wild-type mice treated with Indazole-Cl treated (n=24, black solid line), ERβ−/− mice treated with EtOH (n=8, red dashed line) and ERβ−/− mice treated with Indazole-Cl (n=8, red solid line) (0 = no evidence of disease, 4 = moribund. See Supplemental Experimental Procedures for immunization protocol and scoring system). Data are representative of three independent experiments. C. Indazole–Cl induces partial remission of established EAE. EAE was induced and scored as in B, but Indazole-Cl (n=8) or EtOH vehicle (n=8) treatments were not initiated until mice exhibited a clinical score of 1 (~day 10). Data are representative of two independent experiments.. D. A screen of endogenous steroids identifies ADIOL as a suppressor LPS induction of IL-6 in BV2 cells. *p < 0.01 compared to EtOH treated 6 hr LPS stimulated sample. E. Structure of ADIOL. F. Systemic administration of ADIOL (2.4 µmol/kg/day) blocks the ability of LPS injection (10 mg/kg) to induce IL-6 expression in the substantia nigra (n=5/group). Data are representative of two independent experiments. G. ADIOL (2.4 µmol/kg/day) inhibits development of EAE in wild-type female mice, but not in ERβ knockout female mice. Age matched wild-type mice treated with EtOH (n=8, black dashed line), wild-type mice treated with ADIOL (n=8, black solid line), ERβ−/− mice treated with EtOH (n=8, red dashed line) and ERβ−/− mice treated with ADIOL (n=8, red solid line) were scored for clinical severity as in B. Data are representative of three independent experiments. Error bars represent SD. See also Figure S3.
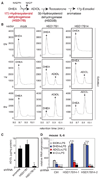
Figure 4. HSD17B14 mediates ADIOL generation in BV2 microglia cells and regulates inflammatory responses
A. A partial scheme of the biosynthesis and metabolism of ADIOL. B. HSD17B14 mediates conversion of DHEA to ADIOL. COS-1 cells were transfected with the indicated expression vectors, using HSD17B1 as a positive control, and the products of DHEA conversion were monitored by gas chromatography at 0, 1 and 6 hr. Retention times for DHEA and ADIOL standards are indicated. C. Knockdown of HSD17B14 in BV2 cells by stable transduction with a lentiviral vector directing expression of a specific shRNA inhibits conversion of DHEA to ADIOL. ADIOL levels in media were quantified 24 hr after addition of DHEA. D. Stable knockdown of HSD17B14 using two different shRNAs blocks the ability of DHEA, but not ADIOL or Indazole-Cl, to suppress LPS induction of IL-6 mRNA in BV2 cells. Cells were pre-treated with the indicated ligands for 1 hour except for DHEA, which was added 6 hours prior to LPS stimulation. *p < 0.01 compared to EtOH treated and LPS stimulated sample. Error bars represent SD. See also Figure S4.
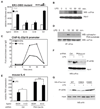
Figure 5. HSD17B expression is regulated by pro- and anti-inflammatory stimuli
A. Knockdown of ERβ and HSD17B14, but not HSD17B1, results in exaggerated and prolonged induction of IL-23p19 mRNA in LPS-treated BV2 cells. B, C. LPS treatment of primary mouse (B) and human (C) microglia cells results in down-regulation of HSD17B14 expression. *p < 0.01 and **p < 0.001 compared to non-stimulated sample. D. LPS treatment of BV2 cells suppresses production of ADIOL. *p < 0.001 compared to non-stimulated sample. E, F. IL-10 induces expression of HSD17B14 mRNA in primary mouse (E) and human (F) microglia. *p < 0.01 compared to non-stimulated sample. G. 17β estradiol (E2) inhibits ADIOL repression of IL6 in LPS-treated BV2 cells. *p < 0.01 compared to ADIOL + LPS alone. In all panels, error bars represent SD. See also Figure S5.
Figure 6. ERβ tethers to cFos in a protein kinase A-dependent manner
A. Sequence-specific DNA binding is not required for ERβ-mediated repression. RAW264.7 cells were transfected with a vector directing expression of a DBD-mutant of ERβ and a specific siRNA directed against ERα. Cells were stimulated with LPS in the presence of the indicated ligands and iNOS-promoter activity was measured by luciferase-reporter assay. *p < 0.01 compared to EtOH treated sample. B. LPS stimulates the interaction of ERβ with cFos in BV2 cells. Lysates of BV2 cells stimulated with LPS for the indicated times were immunoprecipitated with anti-ERβ antibody, and western blots were developed with anti-cFos antibody. C. ERβ is recruited to the IL-23p19 promoter coincident with cFos as detected by ChIP assay. D. LPS treatment of BV2 cells induces phosphorylation of the protein kinase A (PKA) substrate S362 of cFos. E. Protein kinase A is required for Indazole-Cl-dependent repression. Expression of IL-6 mRNA 6hr after LPS stimulation was determined in BV2 cells stably expressing a control shRNA or shRNAs directed against the α and β PKA catalytic subunits. Error bars represent SD. *p < 0.01. F. Knockdown of the PKA-α and β catalytic subunits abolishes LPS-induced interaction of ERβ with cFos as determined by co-immunoprecipitation assay as in D. G. Phosphorylation of cFos at S362 is required for LPS-induced interaction with ERβ. Lentivirus carrying HA-tagged wild-type (WT), S352A and S362E mutant cFos were infected into BV2 cells. Cells were stimulated with LPS for 30 minutes and whole cell extracts were analyzed by immuno-precipitation with α - ERβ antibody and Western blotting for HA. See also Figure S6.
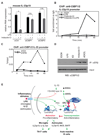
Figure 7. CtBP is a ligand-specific corepressor of ERβ
A. BV2 cells were transfected with specific siRNAs against CtBP1 (siCtBP1), CtBP2 (siCtBP2) or control (siCtrl), and expression of IL-23p19 mRNA was determined 1hr after LPS stimulation. *p < 0.01 compared to siCtrl samples. B. CtBP1/2 are recruited to the IL-23p19 promoter in response to the combination of Indazole-Cl plus LPS (circle, black solid line) as determined by ChIP assay. For Indazole-Cl plus LPS treatment conditions, cells were pre-treated with Indazole-Cl for 1hr followed by LPS for the indicated times. C. Recruitment of CtBP to the IL-23p19 promoter requires ERβ. BV2 cells transduced with shRNA against ERβ (shERβ, solid line) or control (shCtrl, dash line) were pre-treated with Indazole-Cl for 1hr followed by LPS stimulation for the indicated times prior to ChIP assay using anti-CtBP. Data are shown as %input. D. Ligand dependent binding of CtBP and ERβ. BV2 cells were stimulated with LPS, 17β-estradiol (E2), Indazole-Cl and ADIOL for 30 minutes. Lysates were immunoprecipitated with anti-ERβ antibody. IP samples and inputs were developed with anti-CtBP antibody. E. Model for ERβ-mediated repression. See Discussion for details. See also Figure S7.
Comment in
- Estrogen receptor transrepresses brain inflammation.
Gosselin D, Rivest S. Gosselin D, et al. Cell. 2011 May 13;145(4):495-7. doi: 10.1016/j.cell.2011.04.018. Cell. 2011. PMID: 21565607 - Neuroimmunology: estrogen receptor ligands suppress inflammatory responses in astrocytes and microglia.
Wood H. Wood H. Nat Rev Neurol. 2011 Jun 21;7(7):355. doi: 10.1038/nrneurol.2011.87. Nat Rev Neurol. 2011. PMID: 21691333 No abstract available. - Inflammatory disorders: Steroids modulate microglia-mediated inflammation.
Harrison C. Harrison C. Nat Rev Drug Discov. 2011 Jul 1;10(7):492. doi: 10.1038/nrd3485. Nat Rev Drug Discov. 2011. PMID: 21720401 No abstract available.
Similar articles
- 3D models of human ERα and ERβ complexed with 5-androsten-3β,17β-diol.
Baker ME, Uh KY, Chandsawangbhuwana C. Baker ME, et al. Steroids. 2012 Oct;77(12):1192-7. doi: 10.1016/j.steroids.2012.07.014. Epub 2012 Aug 13. Steroids. 2012. PMID: 22921477 - Ameliorative effects of androstenediol against acetic acid-induced colitis in male wistar rats via inhibiting TLR4-mediated PI3K/Akt and NF-κB pathways through estrogen receptor β activation.
Hassan HA, Mohamed Abdelhamid A, Samy W, Osama Mohammed H, Mortada Mahmoud S, Fawzy Abdel Mageed A, Abbas NAT. Hassan HA, et al. Int Immunopharmacol. 2024 Jan 25;127:111414. doi: 10.1016/j.intimp.2023.111414. Epub 2023 Dec 22. Int Immunopharmacol. 2024. PMID: 38141404 - Interaction of Androst-5-ene-3β,17β-diol and 5α-androstane-3β,17β-diol with estrogen and androgen receptors: a combined binding and cell study.
Chen J, Wang WQ, Lin SX. Chen J, et al. J Steroid Biochem Mol Biol. 2013 Sep;137:316-21. doi: 10.1016/j.jsbmb.2013.01.012. Epub 2013 Feb 14. J Steroid Biochem Mol Biol. 2013. PMID: 23416106 - Nudging oligodendrocyte intrinsic signaling to remyelinate and repair: Estrogen receptor ligand effects.
Khalaj AJ, Hasselmann J, Augello C, Moore S, Tiwari-Woodruff SK. Khalaj AJ, et al. J Steroid Biochem Mol Biol. 2016 Jun;160:43-52. doi: 10.1016/j.jsbmb.2016.01.006. Epub 2016 Jan 14. J Steroid Biochem Mol Biol. 2016. PMID: 26776441 Free PMC article. Review. - Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes.
Kwon HS, Koh SH. Kwon HS, et al. Transl Neurodegener. 2020 Nov 26;9(1):42. doi: 10.1186/s40035-020-00221-2. Transl Neurodegener. 2020. PMID: 33239064 Free PMC article. Review.
Cited by
- Targeting estrogen receptor β in microglia and T cells to treat experimental autoimmune encephalomyelitis.
Wu WF, Tan XJ, Dai YB, Krishnan V, Warner M, Gustafsson JÅ. Wu WF, et al. Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3543-8. doi: 10.1073/pnas.1300313110. Epub 2013 Feb 11. Proc Natl Acad Sci U S A. 2013. PMID: 23401502 Free PMC article. - Role of microglia in CNS autoimmunity.
Goldmann T, Prinz M. Goldmann T, et al. Clin Dev Immunol. 2013;2013:208093. doi: 10.1155/2013/208093. Epub 2013 Jun 12. Clin Dev Immunol. 2013. PMID: 23840238 Free PMC article. Review. - Altered Metabolism and DAM-signatures in Female Brains and Microglia with Aging.
Cleland NRW, Potter GJ, Buck C, Quang D, Oldham D, Neal M, Saviola A, Niemeyer CS, Dobrinskikh E, Bruce KD. Cleland NRW, et al. bioRxiv [Preprint]. 2023 Nov 30:2023.11.28.569104. doi: 10.1101/2023.11.28.569104. bioRxiv. 2023. PMID: 38076915 Free PMC article. Updated. Preprint. - Suppression of neuroinflammation by astrocytic dopamine D2 receptors via αB-crystallin.
Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ, Zhou QB, Huang YY, Liu YJ, Wawrousek E, Chen T, Li SB, Xu M, Zhou JN, Hu G, Zhou JW. Shao W, et al. Nature. 2013 Feb 7;494(7435):90-4. doi: 10.1038/nature11748. Epub 2012 Dec 16. Nature. 2013. PMID: 23242137 - Translocator protein 18 kDa negatively regulates inflammation in microglia.
Bae KR, Shim HJ, Balu D, Kim SR, Yu SW. Bae KR, et al. J Neuroimmune Pharmacol. 2014 Jun;9(3):424-37. doi: 10.1007/s11481-014-9540-6. Epub 2014 Apr 1. J Neuroimmune Pharmacol. 2014. PMID: 24687172
References
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. - PubMed
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
- Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145:5021–5032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK015556/DK/NIDDK NIH HHS/United States
- HL087391/HL/NHLBI NIH HHS/United States
- R37 DK015556/DK/NIDDK NIH HHS/United States
- R01 DK015556/DK/NIDDK NIH HHS/United States
- CA52599/CA/NCI NIH HHS/United States
- R01 HL087391/HL/NHLBI NIH HHS/United States
- R01 CA052599/CA/NCI NIH HHS/United States
- R01 DK091183/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases