Generation of induced pluripotent stem cells from human kidney mesangial cells - PubMed (original) (raw)
Generation of induced pluripotent stem cells from human kidney mesangial cells
Bi Song et al. J Am Soc Nephrol. 2011 Jul.
Abstract
Glomerular injury and podocyte loss leads to secondary tubulointerstitial damage and the development of fibrosis. The possibility of genetically reprogramming adult cells, termed induced pluripotent stem cells (iPS), may pave the way for patient-specific stem-cell-based therapies. Here, we reprogrammed normal human mesangial cells to pluripotency by retroviral transduction using defined factors (OCT4, SOX2, KLF4 and c-Myc). The kidney iPS (kiPS) cells resembled human embryonic stem-cell-like colonies in morphology and gene expression: They were alkaline phosphatase-positive; expressed OCT3/4, TRA-1 to 60 and TRA-1 to 81 proteins; and showed downregulation of mesangial cell markers. Quantitative (qPCR) showed that kiPS cells expressed genes analogous to embryonic stem cells and exhibited silencing of the retroviral transgenes by the fourth passage of differentiation. Furthermore, kiPS cells formed embryoid bodies and expressed markers of all three germ layers. The injection of undifferentiated kiPS colonies into immunodeficient mice formed teratomas, thereby demonstrating pluripotency. These results suggest that reprogrammed kidney induced pluripotent stem cells may aid the study of genetic kidney diseases and lead to the development of novel therapies.
Copyright © 2011 by the American Society of Nephrology
Figures
Figure 1.
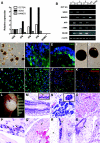
Kidney mesangial cell-derived iPS cells express stem cell markers. (A) The timeline of induction of iPS cells from human mesangial cells following retroviral transduction. (B) Representative images of normal cultured human mesangial cells reprogrammed to generate iPS colonies (C). (D) Alkaline phosphatase-positive iPS colonies (arrows). Immunofluorescence staining of mesangial cell-derived iPS colonies shows localization of OCT3/4 protein (E; green), with corresponding DAPI-stained nuclei (F; blue) and a merged image (G). TRA-1-60 (H–J) and TRA-1-81 (K–M) proteins are also expressed. Original magnifications (B, E–M ×400, C ×10). The graph in the lower panel shows qPCR of mesangial cell markers in NHMCs, with a downregulated expression in iPS cells from 3 separate colonies at passage 4. 120 × 189mm (600 × 600 DPI).
Figure 2.
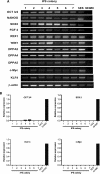
(A) The iPS colonies display a normal 46XY karyotype. (B) PCR of genomic DNA shows the integration of the target vectors in mesangial cell-derived iPS cells at passage 4. Lanes 1–7 show representative iPS cells following retroviral transduction, confirming the expression of OCT3/4, c-Myc, KLF4 and SOX2, compared to human embryonic stem (hES) cells (Lane 8) and normal cultured mesangial cells (NHMC; Lane 9). β-actin is shown as a loading control and for positive amplification. 154x218mm (600 × 600 DPI).
Figure 3.
(A) RT-PCR analysis of stem cell marker genes in kiPS cells for expression of OCT3/4, NANOG, SOX2, FGF4, REX1, TERT, DPPA2, DPPA4, DPPA5, c-Myc and KLF4. Lanes 1–7 represent mesangial cell-derived iPS (kiPS) cells compared to hES cells (Lane 8) and the NHMC target cells at day 0 (Lane 9). The β-actin housekeeping gene is also shown. Panel (B) shows quantitative PCR using primers specific for the transgenes, and not detecting endogenous gene expression levels (shown in Panel A), which confirms retroviral transgene silencing in most kips colonies (passage 4). This is evidenced by a loss of OCT3/4, SOX2, KLF4 and c-Myc expression analogous to expression levels in hES cells. In comparison, an upregulated expression of OCT3/4, SOX2, KLF4 and c-Myc is observed in NHMCS at 6 days following retroviral induction. 112×196mm (150 × 150 DPI).
Figure 4.
Kidney-derived iPS cells demonstrate pluripotency. (A) qPCR of stem cell markers OCT3/4, SOX2 and NANOG in kidney-iPS cells (kiPS) relative to human embryonic stem cells (hES), with an absence of expression in normal human mesangial cells (NHMCs). (B) RT-PCR shows undifferentiated iPS cells (U; Lanes 1, 3, 5) and hES expressed stem cell marker genes OCT3/4, SOX2 and NANOG. In comparison, differentiated embryoid bodies (EBs) (B; Lanes 2, 4, 6) expressed markers from all three germ layers, including α-fetoprotein (AFP; endoderm), α-smooth muscle actin (α-SMA; mesoderm) and neural cell adhesion molecule (NCAM; ectoderm), but did not express undifferentiated stem cell markers. kips formed EBs (C; arrow) with protein localization evident for desmin (D; mesoderm) and Foxa2 (E; endoderm) by immunofluorescence staining. Brightfield images of neural-directed EBs (NDEBs) from hES cells (F) and kiPS cells (G) at day 14. Immunofluorescence staining of NDEBs from hES cells show expression of nestin (H), β-iii-tubulin and MAP2ab (merged, J). In Panels D–K, DAPI-stained nuclei are evident in blue. Nestin (I), β-iii-tubulin and MAP2ab (merged, K) are also observed in NDEBs from kiPS. Original Magnifications C ×10; D, E ×400; F–K ×200. Teratomas are evident following the injection of undifferentiated kiPS cells into immunodeficient mice (L; arrow). Panels M–S show hematoxylin and eosin staining of tissues from all three germ layers, including endoderm; (M) pseudostratified ciliated respiratory epithelium with goblet cells (arrow inset Mag ×1000), (N) serous glands (acini shown in inset Mag ×1000), mesoderm; (O) muscle, (P) nucleated red blood cells (arrows); and endoderm: (Q) neural tissue. At lower power (Mag ×100), panels R and S show glandular tissue (arrows) in cystic teratomas adjacent to adipocytes (asterisk; R) and immature cartilage (asterisk; S). Original magnifications (K × 400; H–J, L ×200; M, N ×100). 120 × 173mm (600 × 600 DPI).
Comment in
- Induced pluripotent stem cells from human kidney.
Rogers I. Rogers I. J Am Soc Nephrol. 2011 Jul;22(7):1179-80. doi: 10.1681/ASN.2011050501. Epub 2011 Jun 16. J Am Soc Nephrol. 2011. PMID: 21680651 No abstract available.
Similar articles
- Inducing pluripotency in somatic cells from the snow leopard (Panthera uncia), an endangered felid.
Verma R, Holland MK, Temple-Smith P, Verma PJ. Verma R, et al. Theriogenology. 2012 Jan 1;77(1):220-8, 228.e1-2. doi: 10.1016/j.theriogenology.2011.09.022. Epub 2011 Nov 11. Theriogenology. 2012. PMID: 22079579 - Generation of induced pluripotent stem cells from buffalo (Bubalus bubalis) fetal fibroblasts with buffalo defined factors.
Deng Y, Liu Q, Luo C, Chen S, Li X, Wang C, Liu Z, Lei X, Zhang H, Sun H, Lu F, Jiang J, Shi D. Deng Y, et al. Stem Cells Dev. 2012 Sep 1;21(13):2485-94. doi: 10.1089/scd.2012.0018. Epub 2012 May 14. Stem Cells Dev. 2012. PMID: 22420535 - The timing of retroviral silencing correlates with the quality of induced pluripotent stem cell lines.
Okada M, Yoneda Y. Okada M, et al. Biochim Biophys Acta. 2011 Feb;1810(2):226-35. doi: 10.1016/j.bbagen.2010.10.004. Epub 2010 Oct 20. Biochim Biophys Acta. 2011. PMID: 20965232 - Emerging methods for preparing iPS cells.
Miyazaki S, Yamamoto H, Miyoshi N, Takahashi H, Suzuki Y, Haraguchi N, Ishii H, Doki Y, Mori M. Miyazaki S, et al. Jpn J Clin Oncol. 2012 Sep;42(9):773-9. doi: 10.1093/jjco/hys108. Epub 2012 Jul 23. Jpn J Clin Oncol. 2012. PMID: 22826352 Review. - Generation of clinically relevant "induced pluripotent stem" (iPS) cells.
Heffernan C, Sumer H, Verma PJ. Heffernan C, et al. J Stem Cells. 2011;6(3):109-27. J Stem Cells. 2011. PMID: 23264997 Review.
Cited by
- C-kit(+) cells isolated from developing kidneys are a novel population of stem cells with regenerative potential.
Rangel EB, Gomes SA, Dulce RA, Premer C, Rodrigues CO, Kanashiro-Takeuchi RM, Oskouei B, Carvalho DA, Ruiz P, Reiser J, Hare JM. Rangel EB, et al. Stem Cells. 2013 Aug;31(8):1644-56. doi: 10.1002/stem.1412. Stem Cells. 2013. PMID: 23733311 Free PMC article. - Three-dimensional imaging of human stem cells using soft X-ray tomography.
Niclis JC, Murphy SV, Parkinson DY, Zedan A, Sathananthan AH, Cram DS, Heraud P. Niclis JC, et al. J R Soc Interface. 2015 Jul 6;12(108):20150252. doi: 10.1098/rsif.2015.0252. J R Soc Interface. 2015. PMID: 26063819 Free PMC article. - Regeneration and bioengineering of the kidney: current status and future challenges.
Salvatori M, Peloso A, Katari R, Orlando G. Salvatori M, et al. Curr Urol Rep. 2014 Jan;15(1):379. doi: 10.1007/s11934-013-0379-9. Curr Urol Rep. 2014. PMID: 24375058 Review. - In vivo maturation of functional renal organoids formed from embryonic cell suspensions.
Xinaris C, Benedetti V, Rizzo P, Abbate M, Corna D, Azzollini N, Conti S, Unbekandt M, Davies JA, Morigi M, Benigni A, Remuzzi G. Xinaris C, et al. J Am Soc Nephrol. 2012 Nov;23(11):1857-68. doi: 10.1681/ASN.2012050505. Epub 2012 Oct 18. J Am Soc Nephrol. 2012. PMID: 23085631 Free PMC article. - The directed differentiation of human iPS cells into kidney podocytes.
Song B, Smink AM, Jones CV, Callaghan JM, Firth SD, Bernard CA, Laslett AL, Kerr PG, Ricardo SD. Song B, et al. PLoS One. 2012;7(9):e46453. doi: 10.1371/journal.pone.0046453. Epub 2012 Sep 28. PLoS One. 2012. PMID: 23029522 Free PMC article.
References
- Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 - PubMed
- Kriz W: Progression of chronic renal failure in focal segmental glomerulosclerosis: Consequence of podocyte damage or of tubulointerstitial fibrosis? Pediatr Nephrol 18: 617–622, 2003 - PubMed
- Ronconi E, Mazzinghi B, Sagrinati C, Angelotti ML, Ballerini L, Parente E, Romagnani P, Lazzeri E, Lasagni L: [The role of podocyte damage in the pathogenesis of glomerulosclerosis and possible repair mechanisms]. G Ital Nefrol 26: 660–669, 2009 - PubMed
- Abbate M, Zoja C, Remuzzi G: How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17: 2974–2984, 2006 - PubMed
- Mundel P, Shankland SJ: Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015, 2002 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous