Reactive oxygen species and hematopoietic stem cell senescence - PubMed (original) (raw)
Reactive oxygen species and hematopoietic stem cell senescence
Lijian Shao et al. Int J Hematol. 2011 Jul.
Abstract
Hematopoietic stem cells (HSCs) are responsible for sustaining hematopoietic homeostasis and regeneration after injury for the entire lifespan of an organism through self-renewal, proliferation, differentiation, and mobilization. Their functions can be affected by reactive oxygen species (ROS) that are produced endogenously through cellular metabolism or after exposure to exogenous stress. At physiological levels, ROS function as signal molecules which can regulate a variety of cellular functions, including HSC proliferation, differentiation, and mobilization. However, an abnormal increase in ROS production occurs under various pathological conditions, which can inhibit HSC self-renewal and induce HSC senescence, resulting in premature exhaustion of HSCs and hematopoietic dysfunction. This review aims to provide a summary of a number of recent findings regarding the cellular sources of ROS in HSCs and the mechanisms of action whereby ROS induce HSC senescence. In particular, we highlight the roles of the p38 mitogen-activated protein kinase (p38)-p16(Ink4a) (p16) pathway in mediating ROS-induced HSC senescence.
Figures
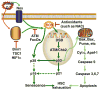
Fig. 1
A hypothetic model for reactive oxygen species (ROS) to mediate the induction of hematopoietic stem cell (HSC) senescence under various pathophysiological conditions. Increased levels of ROS are produced by mitochondria and/or NADPH oxidases (NOXs) in HSCs when HSCs are exposed to stress, a high concentration of oxygen or after deletion of ATM, FoxOs, Bmi1, TSC1, and HIF1α. ROS can induce HSC senescence by activating the ATM-Chk2-p53-p21 pathway via induction of DNA double strand breaks (DSBs) and/or stimulating the p38 pathway. Both pathways converge at p16 for the induction of HSC senescence. In addition, activation of p53 can also induce the expression of various pro-apoptotic proteins to cause HSC apoptosis. ROS may induce HSC senescence and apoptosis in a dose-dependent manner. Both of them can be prevented by treatment with an antioxidant, such as _N_-acetyl-L-cysteine (NAC)
Similar articles
- Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells.
Taniguchi Ishikawa E, Gonzalez-Nieto D, Ghiaur G, Dunn SK, Ficker AM, Murali B, Madhu M, Gutstein DE, Fishman GI, Barrio LC, Cancelas JA. Taniguchi Ishikawa E, et al. Proc Natl Acad Sci U S A. 2012 Jun 5;109(23):9071-6. doi: 10.1073/pnas.1120358109. Epub 2012 May 18. Proc Natl Acad Sci U S A. 2012. PMID: 22611193 Free PMC article. - Polymerase I and transcript release factor transgenic mice show impaired function of hematopoietic stem cells.
Bai L, Lyu Y, Shi G, Li K, Huang Y, Ma Y, Cong YS, Zhang L, Qin C. Bai L, et al. Aging (Albany NY). 2020 Oct 21;12(20):20152-20162. doi: 10.18632/aging.103729. Epub 2020 Oct 21. Aging (Albany NY). 2020. PMID: 33087586 Free PMC article. - [Reactive oxygen species and bone marrow hematopoietic stem cell senescence].
Chai X, Zhao MF. Chai X, et al. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012 Dec;20(6):1518-21. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012. PMID: 23257465 Review. Chinese. - Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells.
Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Ito K, et al. Nat Med. 2006 Apr;12(4):446-51. doi: 10.1038/nm1388. Epub 2006 Mar 26. Nat Med. 2006. PMID: 16565722 - Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells.
Naka K, Muraguchi T, Hoshii T, Hirao A. Naka K, et al. Antioxid Redox Signal. 2008 Nov;10(11):1883-94. doi: 10.1089/ars.2008.2114. Antioxid Redox Signal. 2008. PMID: 18627347 Review.
Cited by
- Bone marrow stromal cells from β-thalassemia patients have impaired hematopoietic supportive capacity.
Crippa S, Rossella V, Aprile A, Silvestri L, Rivis S, Scaramuzza S, Pirroni S, Avanzini MA, Basso-Ricci L, Hernandez RJ, Zecca M, Marktel S, Ciceri F, Aiuti A, Ferrari G, Bernardo ME. Crippa S, et al. J Clin Invest. 2019 Feb 25;129(4):1566-1580. doi: 10.1172/JCI123191. eCollection 2019 Feb 25. J Clin Invest. 2019. PMID: 30830876 Free PMC article. - Bmi-1 plays a critical role in protection from renal tubulointerstitial injury by maintaining redox balance.
Jin J, Lv X, Chen L, Zhang W, Li J, Wang Q, Wang R, Lu X, Miao D. Jin J, et al. Aging Cell. 2014 Oct;13(5):797-809. doi: 10.1111/acel.12236. Epub 2014 Jun 11. Aging Cell. 2014. PMID: 24915841 Free PMC article. - 2, 3, 7, 8-Tetrachlorodibenzo-P-dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms.
Wan C, Liu J, Nie X, Zhao J, Zhou S, Duan Z, Tang C, Liang L, Xu G. Wan C, et al. PLoS One. 2014 Feb 24;9(2):e89811. doi: 10.1371/journal.pone.0089811. eCollection 2014. PLoS One. 2014. PMID: 24587053 Free PMC article. - Increase of circulating IGFBP-4 following genotoxic stress and its implication for senescence.
Alessio N, Squillaro T, Di Bernardo G, Galano G, De Rosa R, Melone MAB, Peluso G, Galderisi U. Alessio N, et al. Elife. 2020 Mar 30;9:e54523. doi: 10.7554/eLife.54523. Elife. 2020. PMID: 32223893 Free PMC article. - Reactive oxygen species and its role in pathogenesis and resistance to therapy in acute myeloid leukemia.
Khorashad JS, Rizzo S, Tonks A. Khorashad JS, et al. Cancer Drug Resist. 2024 Feb 22;7:5. doi: 10.20517/cdr.2023.125. eCollection 2024. Cancer Drug Resist. 2024. PMID: 38434766 Free PMC article. Review.
References
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. - PubMed
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–51. - PubMed
- Ito K, Takubo K, Arai F, Satoh H, Matsuoka S, Ohmura M, Naka K, Azuma M, Miyamoto K, Hosokawa K, Ikeda Y, Mak TW, Suda T, Hirao A. Regulation of reactive oxygen species by Atm is essential for proper response to DNA double-strand breaks in lymphocytes. J Immunol. 2007;178:103–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA086860/CA/NCI NIH HHS/United States
- R01 AI080421/AI/NIAID NIH HHS/United States
- AI080421/AI/NIAID NIH HHS/United States
- R01-CA086688/CA/NCI NIH HHS/United States
- CA102558/CA/NCI NIH HHS/United States
- R01 CA102558/CA/NCI NIH HHS/United States
- R01 CA122023/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical