Mechanisms of membrane transport of folates into cells and across epithelia - PubMed (original) (raw)
Review
Mechanisms of membrane transport of folates into cells and across epithelia
Rongbao Zhao et al. Annu Rev Nutr. 2011.
Abstract
Until recently, the transport of folates into cells and across epithelia has been interpreted primarily within the context of two transporters with high affinity and specificity for folates, the reduced folate carrier and the folate receptors. However, there were discrepancies between the properties of these transporters and characteristics of folate transport in many tissues, most notably the intestinal absorption of folates, in terms of pH dependency and substrate specificity. With the recent cloning of the proton-coupled folate transporter (PCFT) and the demonstration that this transporter is mutated in hereditary folate malabsorption, an autosomal recessive disorder, the molecular basis for this low-pH transport activity is now understood. This review focuses on the properties of PCFT and briefly addresses the two other folate-specific transporters along with other facilitative and ATP-binding cassette (ABC) transporters with folate transport activities. The role of these transporters in the vectorial transport of folates across epithelia is considered.
Figures
Figure 1
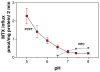
The pH profile of reduced folate carrier (RFC)- and proton-coupled folate transporter (PCFT)-mediated methotrexate (MTX) influx. These data were obtained from wild-type Hela cells and Hela cells in which RFC was deleted from the genome. From Reference .
Figure 2
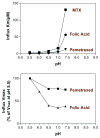
How changes in pH affect folate influx kinetics parameters mediated by the proton-coupled folate transporter (PCFT). (Top panel) The pattern of changes in influx Km is compared among folic acid, methotrexate (MTX), and pemetrexed. (Bottom panel) The pattern of changes in influx Vmax for folic acid and pemetrexed. From References and .
Figure 3
The genomic location, organization, and topology of the proton-coupled folate transporter (PCFT) and residues that play an important role in function. The top panel is the location of PCFT on chromosome 17, the middle panel is the organization of the PCFT gene, and the lower panel is the confirmed PCFT topology. The colored components illustrate how the five exons code for different regions of the protein. Residues highlighted include Glu185 that is required for proton coupling; His281 that plays a role in proton binding and, allosterically, folate binding; His247 and Ser172 (connected by the interrupted line) that appear to be in proximity in the tertiary structure and play a role controlling substrate access to the folate binding pocket through the aqueous translocation pathway; Asp109 and Arg113 in the first intracellular loop that may be required for carrier oscillation between its inward- and outward-facing conformations; Arg376 that plays a role in folate substrate binding; and Asp156 that is important for protein stability. The topological basis for this structure was reported in References , , and .
Figure 4
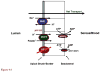
Figure 4**A.** Folate transport across the enterocyte of the proximal small intestine. The pH at the microenvironment of the apical brush-border is 5.8–6.0. The reduced folate carrier (RFC) is expressed at the apical membrane; however, its pH optimum is 7.4. RFC does not contribute significantly to folate transport across this membrane since it cannot compensate for loss of the proton-coupled folate transporter (PCFT) function in subjects with hereditary folate malabsorption (HFM) on a normal diet. The multidrug resistance-associated protein (MRP)3 plays a role in mediating transport of folates across the basolateral membrane of the proximal small intestine. MRP1 and MRP5 are also expressed at this membrane, but their role in transport of folates has not been demonstrated. Not shown is MRP2/ATP-binding cassette (ABC)G2 along with OATPB (SLC21A9) expressed at the apical membrane. Their impact on folate transport at this site is not clear. Figure 4**B.** Folate transport across the choroid plexus. Both the proton-coupled folate transporter (PCFT) and folate receptor α(FRα) are required for transport of folates from blood to cerebrospinal fluid across choroid plexus ependymal cells, based upon studies in human subjects in whom there are loss-of-function mutations in these transporters. However, the bulk of FRα expression, and all reduced folate carrier (RFC) expression, is at the apical brush-border membrane. The pH at the basolateral membrane, where PCFT is expressed, is not known. Not shown are multidrug resistance-associated protein (MRP)1 and MRP4 that are expressed at the basolateral membrane. Their impact on the transport of folates is not clear. Figure 4**C.** Folate transport across the proximal renal tubule. Folate receptor α(FRα) is highly expressed at the apical membrane and plays an important role in the reabsorption of filtered folates. The proton-coupled folate transporter (PCFT) is also highly expressed in the kidney and appears to be expressed in the apical membrane based upon the dominant low-pH transport activity in membrane vesicles from this segment of the kidney; however, its role is not clear. Although the reduced folate carrier (RFC) is located at the basolateral membrane, and could contribute to transport into the peritubular compartment, this transporter favors transport into the cells. Not shown are multidrug resistance-associated protein (MRP)2, MRP4, and ATP-binding cassette (ABC)G2 expressed at the apical membrane, and Oat1–4 expressed at the basolateral membrane. Their contributions relative to the folate-specific transporters is not clear, although one or more of the MRPs may account for secretion of methotrexate.
Figure 4
Figure 4**A.** Folate transport across the enterocyte of the proximal small intestine. The pH at the microenvironment of the apical brush-border is 5.8–6.0. The reduced folate carrier (RFC) is expressed at the apical membrane; however, its pH optimum is 7.4. RFC does not contribute significantly to folate transport across this membrane since it cannot compensate for loss of the proton-coupled folate transporter (PCFT) function in subjects with hereditary folate malabsorption (HFM) on a normal diet. The multidrug resistance-associated protein (MRP)3 plays a role in mediating transport of folates across the basolateral membrane of the proximal small intestine. MRP1 and MRP5 are also expressed at this membrane, but their role in transport of folates has not been demonstrated. Not shown is MRP2/ATP-binding cassette (ABC)G2 along with OATPB (SLC21A9) expressed at the apical membrane. Their impact on folate transport at this site is not clear. Figure 4**B.** Folate transport across the choroid plexus. Both the proton-coupled folate transporter (PCFT) and folate receptor α(FRα) are required for transport of folates from blood to cerebrospinal fluid across choroid plexus ependymal cells, based upon studies in human subjects in whom there are loss-of-function mutations in these transporters. However, the bulk of FRα expression, and all reduced folate carrier (RFC) expression, is at the apical brush-border membrane. The pH at the basolateral membrane, where PCFT is expressed, is not known. Not shown are multidrug resistance-associated protein (MRP)1 and MRP4 that are expressed at the basolateral membrane. Their impact on the transport of folates is not clear. Figure 4**C.** Folate transport across the proximal renal tubule. Folate receptor α(FRα) is highly expressed at the apical membrane and plays an important role in the reabsorption of filtered folates. The proton-coupled folate transporter (PCFT) is also highly expressed in the kidney and appears to be expressed in the apical membrane based upon the dominant low-pH transport activity in membrane vesicles from this segment of the kidney; however, its role is not clear. Although the reduced folate carrier (RFC) is located at the basolateral membrane, and could contribute to transport into the peritubular compartment, this transporter favors transport into the cells. Not shown are multidrug resistance-associated protein (MRP)2, MRP4, and ATP-binding cassette (ABC)G2 expressed at the apical membrane, and Oat1–4 expressed at the basolateral membrane. Their contributions relative to the folate-specific transporters is not clear, although one or more of the MRPs may account for secretion of methotrexate.
Figure 4
Figure 4**A.** Folate transport across the enterocyte of the proximal small intestine. The pH at the microenvironment of the apical brush-border is 5.8–6.0. The reduced folate carrier (RFC) is expressed at the apical membrane; however, its pH optimum is 7.4. RFC does not contribute significantly to folate transport across this membrane since it cannot compensate for loss of the proton-coupled folate transporter (PCFT) function in subjects with hereditary folate malabsorption (HFM) on a normal diet. The multidrug resistance-associated protein (MRP)3 plays a role in mediating transport of folates across the basolateral membrane of the proximal small intestine. MRP1 and MRP5 are also expressed at this membrane, but their role in transport of folates has not been demonstrated. Not shown is MRP2/ATP-binding cassette (ABC)G2 along with OATPB (SLC21A9) expressed at the apical membrane. Their impact on folate transport at this site is not clear. Figure 4**B.** Folate transport across the choroid plexus. Both the proton-coupled folate transporter (PCFT) and folate receptor α(FRα) are required for transport of folates from blood to cerebrospinal fluid across choroid plexus ependymal cells, based upon studies in human subjects in whom there are loss-of-function mutations in these transporters. However, the bulk of FRα expression, and all reduced folate carrier (RFC) expression, is at the apical brush-border membrane. The pH at the basolateral membrane, where PCFT is expressed, is not known. Not shown are multidrug resistance-associated protein (MRP)1 and MRP4 that are expressed at the basolateral membrane. Their impact on the transport of folates is not clear. Figure 4**C.** Folate transport across the proximal renal tubule. Folate receptor α(FRα) is highly expressed at the apical membrane and plays an important role in the reabsorption of filtered folates. The proton-coupled folate transporter (PCFT) is also highly expressed in the kidney and appears to be expressed in the apical membrane based upon the dominant low-pH transport activity in membrane vesicles from this segment of the kidney; however, its role is not clear. Although the reduced folate carrier (RFC) is located at the basolateral membrane, and could contribute to transport into the peritubular compartment, this transporter favors transport into the cells. Not shown are multidrug resistance-associated protein (MRP)2, MRP4, and ATP-binding cassette (ABC)G2 expressed at the apical membrane, and Oat1–4 expressed at the basolateral membrane. Their contributions relative to the folate-specific transporters is not clear, although one or more of the MRPs may account for secretion of methotrexate.
Similar articles
- The proton-coupled folate transporter (PCFT-SLC46A1) and the syndrome of systemic and cerebral folate deficiency of infancy: Hereditary folate malabsorption.
Zhao R, Aluri S, Goldman ID. Zhao R, et al. Mol Aspects Med. 2017 Feb;53:57-72. doi: 10.1016/j.mam.2016.09.002. Epub 2016 Sep 21. Mol Aspects Med. 2017. PMID: 27664775 Free PMC article. Review. - Regulation of folate transport at the mouse arachnoid barrier.
Sangha V, Aboulhassane S, Bendayan R. Sangha V, et al. Fluids Barriers CNS. 2024 Aug 27;21(1):67. doi: 10.1186/s12987-024-00566-0. Fluids Barriers CNS. 2024. PMID: 39192328 Free PMC article. - Biology of the major facilitative folate transporters SLC19A1 and SLC46A1.
Hou Z, Matherly LH. Hou Z, et al. Curr Top Membr. 2014;73:175-204. doi: 10.1016/B978-0-12-800223-0.00004-9. Curr Top Membr. 2014. PMID: 24745983 Free PMC article. Review. - Adaptive transport of folic acid across renal epithelia in folate-deficient rats.
Wani NA, Kaur J. Wani NA, et al. J Physiol Sci. 2012 Nov;62(6):461-8. doi: 10.1007/s12576-012-0223-x. Epub 2012 Aug 4. J Physiol Sci. 2012. PMID: 22865158 Free PMC article. - Folate and thiamine transporters mediated by facilitative carriers (SLC19A1-3 and SLC46A1) and folate receptors.
Zhao R, Goldman ID. Zhao R, et al. Mol Aspects Med. 2013 Apr-Jun;34(2-3):373-85. doi: 10.1016/j.mam.2012.07.006. Mol Aspects Med. 2013. PMID: 23506878 Free PMC article. Review.
Cited by
- Epigenetic alterations in folate transport genes in placental tissue from fetuses with neural tube defects and in leukocytes from subjects with hyperhomocysteinemia.
Farkas SA, Böttiger AK, Isaksson HS, Finnell RH, Ren A, Nilsson TK. Farkas SA, et al. Epigenetics. 2013 Mar;8(3):303-16. doi: 10.4161/epi.23988. Epub 2013 Feb 15. Epigenetics. 2013. PMID: 23417011 Free PMC article. - Supplementation with vitamin D3 during pregnancy protects against lipopolysaccharide-induced neural tube defects through improving placental folate transportation.
Chen YH, Yu Z, Fu L, Xia MZ, Zhao M, Wang H, Zhang C, Hu YF, Tao FB, Xu DX. Chen YH, et al. Toxicol Sci. 2015 May;145(1):90-7. doi: 10.1093/toxsci/kfv036. Epub 2015 Feb 10. Toxicol Sci. 2015. PMID: 25673501 Free PMC article. - Scientific opinion on the tolerable upper intake level for folate.
EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA Panel); Turck D, Bohn T, Castenmiller J, de Henauw S, Hirsch-Ernst KI, Knutsen HK, Maciuk A, Mangelsdorf I, McArdle HJ, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Crous-Bou M, Molloy A, Ciccolallo L, de Sesmaisons Lecarré A, Fabiani L, Horvath Z, Karavasiloglou N, Naska A. EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA Panel), et al. EFSA J. 2023 Nov 13;21(11):e08353. doi: 10.2903/j.efsa.2023.8353. eCollection 2023 Nov. EFSA J. 2023. PMID: 37965303 Free PMC article. - The antifolates.
Visentin M, Zhao R, Goldman ID. Visentin M, et al. Hematol Oncol Clin North Am. 2012 Jun;26(3):629-48, ix. doi: 10.1016/j.hoc.2012.02.002. Hematol Oncol Clin North Am. 2012. PMID: 22520983 Free PMC article. Review. - Structural determinants of human proton-coupled folate transporter oligomerization: role of GXXXG motifs and identification of oligomeric interfaces at transmembrane domains 3 and 6.
Wilson MR, Kugel S, Huang J, Wilson LJ, Wloszczynski PA, Ye J, Matherly LH, Hou Z. Wilson MR, et al. Biochem J. 2015 Jul 1;469(1):33-44. doi: 10.1042/BJ20150169. Epub 2015 Apr 16. Biochem J. 2015. PMID: 25877470 Free PMC article.
References
- Abe T, Unno M, Onogawa T, Tokui T, Kondo TN, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology. 2001;120:1689–99. - PubMed
- Andrews NC. When is a heme transporter not a heme transporter? When it’s a folate transporter. Cell Metab. 2007;5:5–6. - PubMed
- Arias IM, Forgac M. The sinusoidal domain of the plasma membrane of rat hepatocytes contains an amiloride-sensitive Na+/H+ antiport. J Biol Chem. 1984;259:5406–8. - PubMed
- Assaraf YG, Rothem L, Hooijberg JH, Stark M, Ifergan I, et al. Loss of multidrug resistance protein 1 expression and folate efflux activity results in a highly concentrative folate transport in human leukemia cells. J Biol Chem. 2003;278:6680–86. - PubMed
- Assaraf YG, Sierra EE, Babani S, Goldman ID. Inhibitory effects of prostaglandin A1 on membrane transport of folates mediated by both the reduced folate carrier and ATP-driven exporters. Biochem Pharmacol. 1999;58:1321–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases