Isl1 is required for multiple aspects of motor neuron development - PubMed (original) (raw)
Isl1 is required for multiple aspects of motor neuron development
Xingqun Liang et al. Mol Cell Neurosci. 2011 Jul.
Abstract
The LIM homeodomain transcription factor Islet1 (Isl1) is expressed in multiple organs and plays essential roles during embryogenesis. Isl1 is required for the survival and specification of spinal cord motor neurons. Due to early embryonic lethality and loss of motor neurons, the role of Isl1 in other aspects of motor neuron development remains unclear. In this study, we generated Isl1 mutant mouse lines expressing graded doses of Isl1. Our study has revealed essential roles of Isl1 in multiple aspects of motor neuron development, including motor neuron cell body localization, motor column formation and axon growth. In addition, Isl1 is required for survival of cranial ganglia neurons.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
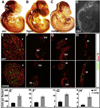
Fig. 1
Reduced Isl1 expression in regions of the central nervous system in Isl1 compound mutant. Isl1 is expressed in various regions of the central nervous system, including the outer layer of the striatum (St) and diencephalon (Di) (A, B, I), the motor neurons (MNs) of the ventral hindbrain (A, B, C, K), cranial ganglia/nucleus (shown are V, VII–XII) (A, B, J) and the spinal MNs (A, D, L) and dI3 interneurons (dI3 IN) (D). In Isl1 compound mutants (comp), the level of Isl1 expression and the number of Isl1 expressing cells are significantly reduced as revealed by Xgal staining (E–H) and Isl1 antibody staining (M–P, comp).
Fig. 2
Reduced Isl1 expression in Isl1 compound mutant leads to increased apoptosis and abnormal development of cranial ganglia. Wholemount neurofilament staining of control (ctrl) (A), Isl1 hypomorphic (hypo) (B), Isl1 compound mutant (comp) (C) and Nestin-Cre; Isl1 conditional knockout (cko) (D) embryos at E11.5. Compared to control embryos, Isl1 hypomorphic mutant embryos displayed a relative normal size of cranial ganglia III, V, VII/VIII, X and XI (B). However, ectopic axonal projections from the cranial ganglion IX were observed (B, white arrow). Axonal projections of XI and XII ganglia were significantly reduced and blunted (B, bracket and arrowhead). In Isl1 compound mutant (C), further reduction in Isl1 expression led to reductions in the size of the cranial ganglia (V, VII/VIII, IX and X). Reductions in axon projections were observed in ganglia V (ophthalmic, maxillary and mandibular branches), VII, IX, X, XI and XII (C). Nestin-Cre; Isl1 conditional knockout embryos displayed an overall developmental defect with severe reduction in the size of cranial ganglia and their axon projections (D). TUNEL staining at E11.5 revealed significantly increased apoptosis in the cranial ganglia V, VII, IX and XI of Isl1 compound mutant compared to control embryo (E–H and E′–H′).
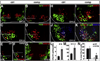
Fig. 3
Decreased MN genesis in Isl1 compound mutant embryos. At E9 (18 Somites), a few Isl1 expressing MNs are generated in the anterior segments of the control spinal cord, a subpopulation of which coexpress Hb9 (A). However, in Isl1 compound mutant spinal cord, significantly fewer Isl1 expressing MNs are generated and none of which expresses Hb9 (B). At E11.5, compared to that of control mice (C, E), there is a dramatic reduction in the number of MNs marked by the expression of Isl1, Isl2 and Hb9 in Isl1 compound mutant (D, F). At E11.5, Lhx1 expression in the spinal cord of Isl1 compound mutant is comparable to that of control embryo (G, H). Lhx3 is expressed in V2 INs, and the MNs of the medial motor column where it is coexpressed with HB9 (I). However, the number of Lhx3 expressing cells in the spinal cord of Isl1 compound mutant embryos is significantly increased at E11.5 (J, L—red). Ectopic Lhx3 expressing cells were observed to invade into more ventrolateral region, a few of which coexpress HB9 (J). However, at E10.5 the number of Lhx3 expressing cells is comparable in mutant and control samples (K, L—blue).
Fig. 4
Reduced Isl1 expression leads to conversion of prospective MNs to V2a INs. A significant increase in the number of Chx10 expressing V2a interneurons (INs) was observed in the spinal cord of Isl1 compound mutant embryos at E11.5 (A–D, M). These Chx10 cells found ectopically in more ventrolateral position, mixed with remaining MNs (B, D, below the bar). At E10, a few Chx10+ V2a INs start to emerge in the domain dorsal to that of Hb9+ MNs (E). A significant increase in the number of Chx10+ INs was readily detectable at this stage, which assumed a more ventral position and was mixed with Hb9 MNs (F, L). Some mutant cells started to express Chx10 immediately after they left the ventricular zone and started to differentiate (F, arrowhead). No coexpression of V2a IN marker Chx10 with MN marker Isl1 (A, B) and Hb9 (C–F) were observed in both Isl1 compound mutant and control embryos. Chx10 is never expressed in Isl1 lineage (YFP) in the normal spinal cord (G). Isl1-βgal is not coexpressed with Chx10 in control embryos (H). However, a significant number of ventral ectopic Chx10 V2a INs in Isl1 compound mutant embryos coexpress Isl1-βgal (45.4+/−23.4 per section) (I). Intraspinal cord labeling revealed that Chx10 V2a INs were similarly labeled by Rhodamine-dextran (Rhd) in both control (16.6 ± 0.5% of total Chx10+ cells) and Isl1 compound mutant (17.2 ± 3.44%) (J, K, arrowhead and N-a). A significant number of ectopic V2a INs in Isl1 compound mutant, marked by the coexpression of Chx10 and Isl1-βgal, were also labeled by Rhodamine-dextran (K-arrow and N-b). a: Rhd+/Chx10+; b: Rhd+/Chx10+/βgal+.
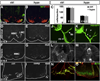
Fig. 5
Defects in MN migration, motor column formation and axon growth in Isl1 hypomorphic embryos. Despite significant reduction in Isl1 expression in spinal MNs of Isl1 hypomorphic mutant, the level of Hb9 expression and the number of Hb9 expressing MNs during development are not changed (A, B and C). At E16.5, Hb9+ MNs are segregated into medial (MMC) and lateral (LMC) motor columns at the cervical spinal cord, or MMC at the thoracic spinal cord of control mice (D, F). However, in Isl1 hypomorphic embryos, Hb9+ MNs are scattered and ectopically positioned or abnormally clustered and fail to form distinct motor columns (E, G). In Isl1 compound mutant spinal cord, MNs were frequently observed to migrate along ventral roots out of neural tube (H, I arrow). In Hb9-GFP transgenic mice, GFP expression marks the axons of the spinal MNs, which after exit from vertebral column, are divided into dorsal branch (db) and ventral branch (vb) (J). In Isl1 hypomorphic mice that have been crossed onto Hb9-GFP transgenic background, the dorsal branch of the spinal motor axons appeared to be blunt (K, arrow) and frequent ectopic motor projections were observed (K, arrowhead). Wholemount neurofilament immunostaining and Alexa 488-α-bungarotoxin (BTX) staining revealed motor innervation of diaphragm by phrenic nerve and the formation of neuromuscular junctions on the diaphragm of control mice (L, N). However, diaphragm of Isl1 hypomorphic mutant mice were deinnervated and no functional neuromuscular junctions were detected (M, O).
Similar articles
- A postmitotic role for Isl-class LIM homeodomain proteins in the assignment of visceral spinal motor neuron identity.
Thaler JP, Koo SJ, Kania A, Lettieri K, Andrews S, Cox C, Jessell TM, Pfaff SL. Thaler JP, et al. Neuron. 2004 Feb 5;41(3):337-50. doi: 10.1016/s0896-6273(04)00011-x. Neuron. 2004. PMID: 14766174 - Critical Roles of the LIM Domains of Lhx3 in Recruiting Coactivators to the Motor Neuron-Specifying Isl1-Lhx3 Complex.
Seo SY, Lee B, Lee S. Seo SY, et al. Mol Cell Biol. 2015 Oct;35(20):3579-89. doi: 10.1128/MCB.00335-15. Epub 2015 Aug 10. Mol Cell Biol. 2015. PMID: 26260513 Free PMC article. - Functional Diversification of Motor Neuron-specific Isl1 Enhancers during Evolution.
Kim N, Park C, Jeong Y, Song MR. Kim N, et al. PLoS Genet. 2015 Oct 8;11(10):e1005560. doi: 10.1371/journal.pgen.1005560. eCollection 2015 Oct. PLoS Genet. 2015. PMID: 26447474 Free PMC article. - Together at last: bHLH and LIM-HD regulators cooperate to specify motor neurons.
Allan DW, Thor S. Allan DW, et al. Neuron. 2003 Jun 5;38(5):675-7. doi: 10.1016/s0896-6273(03)00329-5. Neuron. 2003. PMID: 12797950 Review. - Transcriptional codes and the control of neuronal identity.
Shirasaki R, Pfaff SL. Shirasaki R, et al. Annu Rev Neurosci. 2002;25:251-81. doi: 10.1146/annurev.neuro.25.112701.142916. Epub 2002 Mar 27. Annu Rev Neurosci. 2002. PMID: 12052910 Review.
Cited by
- ISL1 is necessary for auditory neuron development and contributes toward tonotopic organization.
Filova I, Pysanenko K, Tavakoli M, Vochyanova S, Dvorakova M, Bohuslavova R, Smolik O, Fabriciova V, Hrabalova P, Benesova S, Valihrach L, Cerny J, Yamoah EN, Syka J, Fritzsch B, Pavlinkova G. Filova I, et al. Proc Natl Acad Sci U S A. 2022 Sep 13;119(37):e2207433119. doi: 10.1073/pnas.2207433119. Epub 2022 Sep 8. Proc Natl Acad Sci U S A. 2022. PMID: 36074819 Free PMC article. - Landscape of histone modifications in a sponge reveals the origin of animal _cis_-regulatory complexity.
Gaiti F, Jindrich K, Fernandez-Valverde SL, Roper KE, Degnan BM, Tanurdžić M. Gaiti F, et al. Elife. 2017 Apr 11;6:e22194. doi: 10.7554/eLife.22194. Elife. 2017. PMID: 28395144 Free PMC article. - Spinal Cord Organoids to Study Motor Neuron Development and Disease.
Buchner F, Dokuzluoglu Z, Grass T, Rodriguez-Muela N. Buchner F, et al. Life (Basel). 2023 May 25;13(6):1254. doi: 10.3390/life13061254. Life (Basel). 2023. PMID: 37374039 Free PMC article. Review. - The Efficiency of Direct Maturation: the Comparison of Two hiPSC Differentiation Approaches into Motor Neurons.
Schaefers C, Rothmiller S, Thiermann H, Rein T, Schmidt A. Schaefers C, et al. Stem Cells Int. 2022 Dec 9;2022:1320950. doi: 10.1155/2022/1320950. eCollection 2022. Stem Cells Int. 2022. PMID: 36530489 Free PMC article. - Transcriptional control of motor pool formation and motor circuit connectivity by the LIM-HD protein Isl2.
Lee Y, Yeo IS, Kim N, Lee DK, Kim KT, Yoon J, Yi J, Hong YB, Choi BO, Kosodo Y, Kim D, Park J, Song MR. Lee Y, et al. Elife. 2023 Oct 23;12:e84596. doi: 10.7554/eLife.84596. Elife. 2023. PMID: 37869988 Free PMC article.
References
- Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. - PubMed
- Appel B, Korzh V, Glasgow E, Thor S, Edlund T, Dawid IB, Eisen JS. Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development. 1995;121:4117–4125. - PubMed
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. - PubMed
- Begbie J, Graham A. Integration between the epibranchial placodes and the hindbrain. Science. 2001;294:595–598. - PubMed
- Broihier HT, Skeath JB. Drosophila homeodomain protein dHb9 directs neuronal fate via crossrepressive and cell-nonautonomous mechanisms. Neuron. 2002;35:39–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL070867/HL/NHLBI NIH HHS/United States
- R01 NS037116/NS/NINDS NIH HHS/United States
- R01 NS054172/NS/NINDS NIH HHS/United States
- 5R01HL070867-04/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases