Response of human prostate cancer cells and tumors to combining PARP inhibition with ionizing radiation - PubMed (original) (raw)
Response of human prostate cancer cells and tumors to combining PARP inhibition with ionizing radiation
Juan Camilo Barreto-Andrade et al. Mol Cancer Ther. 2011 Jul.
Abstract
Radiation therapy remains a promising modality for curative treatment of localized prostate cancer, but dose-limiting toxicities significantly limit its effectiveness. Agents that enhance efficacy at lower radiation doses might have considerable value in increasing tumor control without compromising organ function. Here, we tested the hypothesis that the PARP inhibitor ABT-888 (veliparib) can enhance the response of prostate cancer cells and tumors to ionizing radiation (IR). Following exposure of DU-145 and PC-3 prostate cancer cell lines to the combination of 10 μmol/L ABT-888 and 6 Gy, we observed similar persistence between both cell lines of DNA damage foci and in vitro radiosensitization. We have previously observed that persistent DNA damage foci formed after ABT-888 plus IR efficiently promote accelerated cell senescence, but only PC-3 cells displayed the expected senescent response of G(2)-M arrest, induction of p21 and β-galactosidase expression, and accumulation as large flat cells. In turn, combining ABT-888 with 6 Gy resulted in delayed tumor regrowth compared with either agent alone only in PC-3 xenograft tumors, whereas DU-145 tumors continued to grow. By 7 days after treatment with ABT-888 plus IR, PC-3 tumors contained abundant senescent cells displaying persistent DNA damage foci, but no evidence of senescence was noted in the DU-145 tumors. That equivalent radiosensitization by ABT-888 plus IR in vitro failed to predict comparable results with tumors in vivo suggests that the efficacy of PARP inhibitors may partially depend on a competent senescence response to accumulated DNA damage.
© 2011 American Association for Cancer Research.
Conflict of interest statement
Conflicts of interest: None of the authors has potential conflicts of interest.
Figures
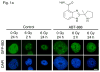
Figure 1
(a) PC-3 GFP-IBD cells show pan-nuclear fluorescence before IR treatment but display ionizing radiation induced foci (IRIF) at 2 h after irradiation that partially resolve by 24 h. The PARP inhibitor ABT-888 induced DNA damage foci on its own. Addition of ABT-888 to IR markedly increased IRIF persistence at 24 h. Bar, 10 μm. (b) Immunofluorescence staining of PC-3 and DU-145 cells after treatment with ABT-888 ± IR. Both cells lines display few γH2AX and 53BP1 foci without ABT-888 or IR treatment. 6 Gy induces similar numbers of γH2AX and 53BP1 foci in each cell line at 2 h, which partially resolve by 24 h. As observed with GFP-IBD, ABT-888 alone induced both γH2AX and 53BP1 foci in PC-3 cells, with no similar effect observed with DU-145 cells. The addition of ABT-888 to IR increased γH2AX and 53BP1 foci persistence at 24 h in both cell lines. Bar, 10 μm.
Figure 1
(a) PC-3 GFP-IBD cells show pan-nuclear fluorescence before IR treatment but display ionizing radiation induced foci (IRIF) at 2 h after irradiation that partially resolve by 24 h. The PARP inhibitor ABT-888 induced DNA damage foci on its own. Addition of ABT-888 to IR markedly increased IRIF persistence at 24 h. Bar, 10 μm. (b) Immunofluorescence staining of PC-3 and DU-145 cells after treatment with ABT-888 ± IR. Both cells lines display few γH2AX and 53BP1 foci without ABT-888 or IR treatment. 6 Gy induces similar numbers of γH2AX and 53BP1 foci in each cell line at 2 h, which partially resolve by 24 h. As observed with GFP-IBD, ABT-888 alone induced both γH2AX and 53BP1 foci in PC-3 cells, with no similar effect observed with DU-145 cells. The addition of ABT-888 to IR increased γH2AX and 53BP1 foci persistence at 24 h in both cell lines. Bar, 10 μm.
Figure 2
(a) Effect of ABT-888 treatment alone on colony formation in PC-3 and DU-145 cell lines. Significant growth inhibition was observed only in PC-3 cells. (b) Clonogenic survival of PC-3 and DU-145 cells after treatment with IR doses from 0 to 6 Gy, with or without 10 μM ABT-888. Although the PARP inhibitor sensitizes both cell lines to radiation, the effect is more significant in the PC-3 cells. Bars, SD.
Figure 2
(a) Effect of ABT-888 treatment alone on colony formation in PC-3 and DU-145 cell lines. Significant growth inhibition was observed only in PC-3 cells. (b) Clonogenic survival of PC-3 and DU-145 cells after treatment with IR doses from 0 to 6 Gy, with or without 10 μM ABT-888. Although the PARP inhibitor sensitizes both cell lines to radiation, the effect is more significant in the PC-3 cells. Bars, SD.
Figure 3
Cell cycle analysis of PC-3 and DU-145 cells after treatment with ABT-888 and IR alone or in combination. Propidium iodide flow cytometry was performed 48 h after treatment with ABT-888 ± IR and cell cycle statistics were modeled with FlowJo. 48 h incubation in 10 μM ABT-888 increased the G2/M fraction in PC-3 cells but had no effect on DU-145 cells. Irradiation with 6 Gy decreased S phase and increased the G2/M fraction in both cell lines. This effect was enhanced by ABT-888 in each. The combined effect of ABT-888 and IR on PC-3 cells also markedly depleted G1 phase cells from the population, suggesting a G2 cell cycle arrest.
Figure 4
Accelerated senescence in DU-145 and PC-3 cells induced by ABT-888 and IR alone or in combination. (a) Analysis of SA-βGal activity 7 d after treatment showed that 6 Gy with or without 10 μM ABT-888 increased SA-βGal activity in PC-3 cells. IR alone had less effect on DU-145 cells, which showed some increase in SA-βGal staining with IR + ABT-888. Bar, 20 μm. (b) qPCR analysis of p21 expression demonstrated a significant increase only in PC-3 cells 48 h after 6 Gy with 10 μM ABT-888. Bars, SD. (c) Immunofluorescence analysis at 72 h after treatment showed significant accumulation of p21 protein only in PC-3 cells treated with both IR and ABT-888. Bar, 10 μm.
Figure 4
Accelerated senescence in DU-145 and PC-3 cells induced by ABT-888 and IR alone or in combination. (a) Analysis of SA-βGal activity 7 d after treatment showed that 6 Gy with or without 10 μM ABT-888 increased SA-βGal activity in PC-3 cells. IR alone had less effect on DU-145 cells, which showed some increase in SA-βGal staining with IR + ABT-888. Bar, 20 μm. (b) qPCR analysis of p21 expression demonstrated a significant increase only in PC-3 cells 48 h after 6 Gy with 10 μM ABT-888. Bars, SD. (c) Immunofluorescence analysis at 72 h after treatment showed significant accumulation of p21 protein only in PC-3 cells treated with both IR and ABT-888. Bar, 10 μm.
Figure 4
Accelerated senescence in DU-145 and PC-3 cells induced by ABT-888 and IR alone or in combination. (a) Analysis of SA-βGal activity 7 d after treatment showed that 6 Gy with or without 10 μM ABT-888 increased SA-βGal activity in PC-3 cells. IR alone had less effect on DU-145 cells, which showed some increase in SA-βGal staining with IR + ABT-888. Bar, 20 μm. (b) qPCR analysis of p21 expression demonstrated a significant increase only in PC-3 cells 48 h after 6 Gy with 10 μM ABT-888. Bars, SD. (c) Immunofluorescence analysis at 72 h after treatment showed significant accumulation of p21 protein only in PC-3 cells treated with both IR and ABT-888. Bar, 10 μm.
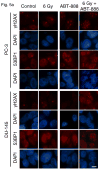
Figure 5
Foci persistence and accelerated senescence in PC-3 and DU-145 tumors treated with ABT-888 and IR alone or in combination. (a) Immunofluorescence staining in PC-3 and DU-145 tissue sections from tumors harvested 4 d after treatment with ABT-888 ± IR. Both cell lines display small numbers of γH2AX and endogenous 53BP1 nuclear foci 4 d after 6 Gy alone. ABT-888 increased the number of persistent foci in PC-3 but had a less marked effect on DU-145 tumors. Bar, 10 μm. (b) Tumors excised at 7 d after treatment, sectioned and analyzed for SA-βGal display increased activity in PC-3 tumors after treatment with IR + ABT-888. DU-145 tumors show only isolated cells with SA-βGal staining. Bar, 20 μm.
Figure 5
Foci persistence and accelerated senescence in PC-3 and DU-145 tumors treated with ABT-888 and IR alone or in combination. (a) Immunofluorescence staining in PC-3 and DU-145 tissue sections from tumors harvested 4 d after treatment with ABT-888 ± IR. Both cell lines display small numbers of γH2AX and endogenous 53BP1 nuclear foci 4 d after 6 Gy alone. ABT-888 increased the number of persistent foci in PC-3 but had a less marked effect on DU-145 tumors. Bar, 10 μm. (b) Tumors excised at 7 d after treatment, sectioned and analyzed for SA-βGal display increased activity in PC-3 tumors after treatment with IR + ABT-888. DU-145 tumors show only isolated cells with SA-βGal staining. Bar, 20 μm.
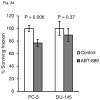
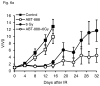
Figure 6
PC-3 and DU-145 tumor growth kinetics after treatment with ABT-888 and IR alone or in combination. Tumor volume was followed over time in PC-3 (a) and DU-145 (b) xenografts in animals treated with ABT-888 followed by IR. PC-3 tumors showed minimal sensitivity to ABT-888 treatment alone but significantly delay in tumor regrowth after 6 Gy with the addition of ABT-888. DU-145 tumor growth was not significantly slowed by the 6 Gy dose and addition of ABT-888 appeared to have a slight protective effect.
Figure 6
PC-3 and DU-145 tumor growth kinetics after treatment with ABT-888 and IR alone or in combination. Tumor volume was followed over time in PC-3 (a) and DU-145 (b) xenografts in animals treated with ABT-888 followed by IR. PC-3 tumors showed minimal sensitivity to ABT-888 treatment alone but significantly delay in tumor regrowth after 6 Gy with the addition of ABT-888. DU-145 tumor growth was not significantly slowed by the 6 Gy dose and addition of ABT-888 appeared to have a slight protective effect.
Similar articles
- Poly(ADP-ribose) polymerase inhibitor induces accelerated senescence in irradiated breast cancer cells and tumors.
Efimova EV, Mauceri HJ, Golden DW, Labay E, Bindokas VP, Darga TE, Chakraborty C, Barreto-Andrade JC, Crawley C, Sutton HG, Kron SJ, Weichselbaum RR. Efimova EV, et al. Cancer Res. 2010 Aug 1;70(15):6277-82. doi: 10.1158/0008-5472.CAN-09-4224. Epub 2010 Jul 7. Cancer Res. 2010. PMID: 20610628 Free PMC article. - Radiosensitization by PARP Inhibition in DNA Repair Proficient and Deficient Tumor Cells: Proliferative Recovery in Senescent Cells.
Alotaibi M, Sharma K, Saleh T, Povirk LF, Hendrickson EA, Gewirtz DA. Alotaibi M, et al. Radiat Res. 2016 Mar;185(3):229-45. doi: 10.1667/RR14202.1. Epub 2016 Mar 2. Radiat Res. 2016. PMID: 26934368 Free PMC article. - ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models.
Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, Bontcheva-Diaz VD, Cox BF, DeWeese TL, Dillehay LE, Ferguson DC, Ghoreishi-Haack NS, Grimm DR, Guan R, Han EK, Holley-Shanks RR, Hristov B, Idler KB, Jarvis K, Johnson EF, Kleinberg LR, Klinghofer V, Lasko LM, Liu X, Marsh KC, McGonigal TP, Meulbroek JA, Olson AM, Palma JP, Rodriguez LE, Shi Y, Stavropoulos JA, Tsurutani AC, Zhu GD, Rosenberg SH, Giranda VL, Frost DJ. Donawho CK, et al. Clin Cancer Res. 2007 May 1;13(9):2728-37. doi: 10.1158/1078-0432.CCR-06-3039. Clin Cancer Res. 2007. PMID: 17473206 - Radiosensitization in prostate cancer: mechanisms and targets.
Palacios DA, Miyake M, Rosser CJ. Palacios DA, et al. BMC Urol. 2013 Jan 26;13:4. doi: 10.1186/1471-2490-13-4. BMC Urol. 2013. PMID: 23351141 Free PMC article. Review. - Crosstalk between immune checkpoint and DNA damage response inhibitors for radiosensitization of tumors.
Classen S, Petersen C, Borgmann K. Classen S, et al. Strahlenther Onkol. 2023 Dec;199(12):1152-1163. doi: 10.1007/s00066-023-02103-8. Epub 2023 Jul 7. Strahlenther Onkol. 2023. PMID: 37420037 Free PMC article. Review.
Cited by
- Moving the Needle Forward in Genomically-Guided Precision Radiation Treatment.
Tam A, Mercier BD, Thomas RM, Tizpa E, Wong IG, Shi J, Garg R, Hampel H, Gray SW, Williams T, Bazan JG, Li YR. Tam A, et al. Cancers (Basel). 2023 Nov 7;15(22):5314. doi: 10.3390/cancers15225314. Cancers (Basel). 2023. PMID: 38001574 Free PMC article. Review. - Molecular Targeted Therapies in Metastatic Prostate Cancer: Recent Advances and Future Challenges.
Sorrentino C, Di Carlo E. Sorrentino C, et al. Cancers (Basel). 2023 May 23;15(11):2885. doi: 10.3390/cancers15112885. Cancers (Basel). 2023. PMID: 37296848 Free PMC article. Review. - PARP-1 genetic polymorphism associated with radiation sensitivity of non-small cell lung cancer.
Wang H, Xie H, Wang S, Zhao J, Gao Y, Chen J, Zhao Y, Guo G. Wang H, et al. Pathol Oncol Res. 2022 Dec 14;28:1610751. doi: 10.3389/pore.2022.1610751. eCollection 2022. Pathol Oncol Res. 2022. PMID: 36590386 Free PMC article. - Distinct mechanisms mediating therapy-induced cellular senescence in prostate cancer.
Kallenbach J, Atri Roozbahani G, Heidari Horestani M, Baniahmad A. Kallenbach J, et al. Cell Biosci. 2022 Dec 15;12(1):200. doi: 10.1186/s13578-022-00941-0. Cell Biosci. 2022. PMID: 36522745 Free PMC article. Review. - From Therapy Resistance to Targeted Therapies in Prostate Cancer.
Moreira-Silva F, Henrique R, Jerónimo C. Moreira-Silva F, et al. Front Oncol. 2022 May 24;12:877379. doi: 10.3389/fonc.2022.877379. eCollection 2022. Front Oncol. 2022. PMID: 35686097 Free PMC article. Review.
References
- Zelefsky MJ, Chan H, Hunt M, Yamada Y, Shippy AM, Amols H. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176:1415–9. - PubMed
- Bill-Axelson A, Holmberg L, Ruutu M, Haggman M, Andersson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–84. - PubMed
- Van Hemelrijck M, Garmo H, Holmberg L, Ingelsson E, Bratt O, Bill-Axelson A, et al. Absolute and relative risk of cardiovascular disease in men with prostate cancer: results from the Population-Based PCBaSe Sweden. J Clin Oncol. 2010;28:3448–56. - PubMed
- Ljungman M. Targeting the DNA damage response in cancer. Chem Rev. 2009;109:2929–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM60443/GM/NIGMS NIH HHS/United States
- R21 CA138365/CA/NCI NIH HHS/United States
- R01 CA111423-05/CA/NCI NIH HHS/United States
- R01 CA111423/CA/NCI NIH HHS/United States
- P50 CA090386-09/CA/NCI NIH HHS/United States
- R21 CA138365-03/CA/NCI NIH HHS/United States
- R01 GM060443/GM/NIGMS NIH HHS/United States
- P50 CA090386/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical