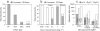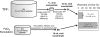A simple and efficient method for concentration of ocean viruses by chemical flocculation - PubMed (original) (raw)
A simple and efficient method for concentration of ocean viruses by chemical flocculation
Seth G John et al. Environ Microbiol Rep. 2011 Apr.
Free PMC article
Abstract
Ocean viruses alter ecosystems through host mortality, horizontal gene transfer and by facilitating remineralization of limiting nutrients. However, the study of wild viral populations is limited by inefficient and unreliable concentration techniques. Here, we develop a new technique to recover viruses from natural waters using iron-based flocculation and large-pore-size filtration, followed by resuspension of virus-containing precipitates in a pH 6 buffer. Recovered viruses are amenable to gene sequencing, and a variable proportion of phages, depending upon the phage, retain their infectivity when recovered. This Fe-based virus flocculation, filtration and resuspension method (FFR) is efficient (> 90% recovery), reliable, inexpensive and adaptable to many aspects of marine viral ecology and genomics research.
Figures
Fig. 1
Optimization of virus concentration and redissolution from Biosphere 2 Ocean viral-fraction seawater.A. The effect of various filters on Fe-virus concentrate recovery after flocculation with 1 mg l−1 Fe: PC = 0.8 µm polycarbonate filters (Whatman Nuclepore), PES = 0.8 µm polyethersulfone (Pall Supor), MCE = 1.2 µm mixed cellulose ester (Millipore RAWP), and GF/B = 1.0 µm nominal pore size glass fibre filters (Whatman).B. The effect of Fe addition on Fe-virus concentrate recovery by filtration onto a polycarbonate membrane or settling.C. The effect of pH and resuspension buffer on the time required for dissolution of the iron hydroxide flocculate. Resuspension buffers were tested with 0.2 M EDTA in all solutions and the addition of either 0.1 M ascorbate or 0.1 M oxalate to two treatments. One millilitre of buffer was used to dissolve 1 mg of Fe.
Fig. 2
Comparison of viral concentration methods showing the experimental design schematic and resulting concentration efficiency using viral-fraction (< 0.22 µm filtrate) natural seawater from Scripps Pier in San Diego, CA. Recovery is based on virus counts by epifluorescence microscopy.
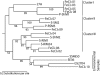
Fig. 3
Phylogenetic tree of previously published gene 20 (myovirus portal protein gene) sequences and gene sequences obtained by PCR amplification of gene 20 from Fe-virus concentrates collected at Scripps Pier. Gene sequences obtained in this study are designated as ‘FeCl3’.
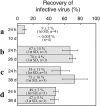
Fig. 4
Infectivity of FeCl3-flocculated viruses after variable durations of storage (24 h to 38 days) and with different resuspension buffers. Infectivity was assessed by agar overlay plaque assay of flocculated and resuspended virus. Recovery was determined for (A) myovirus resuspended in ascorbate buffer, (B) myovirus resuspended in oxalate buffer and immediately transferred to modified SM buffer for long term storage, (C) myovirus resuspended in oxalate buffer, and (D) siphovirus resuspended in oxalate buffer. Viruses in (A) and (B) were spiked into artificial seawater prior to concentration, while viruses in (C) and (D) were spiked into aged natural seawater prior to concentration.
Similar articles
- Iron Chloride Flocculation of Bacteriophages from Seawater.
Poulos BT, John SG, Sullivan MB. Poulos BT, et al. Methods Mol Biol. 2018;1681:49-57. doi: 10.1007/978-1-4939-7343-9_4. Methods Mol Biol. 2018. PMID: 29134586 - Method evaluation for viruses in activated sludge: Concentration, sequencing, and identification.
Zhang Y, Zheng X, Yan W, Wang D, Chen X, Wang Y, Zhang T. Zhang Y, et al. Sci Total Environ. 2024 Dec 10;955:176886. doi: 10.1016/j.scitotenv.2024.176886. Epub 2024 Oct 15. Sci Total Environ. 2024. PMID: 39419205 - Inactivation of F-specific bacteriophages during flocculation with polyaluminum chloride - a mechanistic study.
Kreißel K, Bösl M, Hügler M, Lipp P, Franzreb M, Hambsch B. Kreißel K, et al. Water Res. 2014 Mar 15;51:144-51. doi: 10.1016/j.watres.2013.12.026. Epub 2013 Dec 28. Water Res. 2014. PMID: 24429100 - Ecology of prokaryotic viruses.
Weinbauer MG. Weinbauer MG. FEMS Microbiol Rev. 2004 May;28(2):127-81. doi: 10.1016/j.femsre.2003.08.001. FEMS Microbiol Rev. 2004. PMID: 15109783 Review. - Metaviromics coupled with phage-host identification to open the viral 'black box'.
Moon K, Cho JC. Moon K, et al. J Microbiol. 2021 Mar;59(3):311-323. doi: 10.1007/s12275-021-1016-9. Epub 2021 Feb 23. J Microbiol. 2021. PMID: 33624268 Review.
Cited by
- Global morphological analysis of marine viruses shows minimal regional variation and dominance of non-tailed viruses.
Brum JR, Schenck RO, Sullivan MB. Brum JR, et al. ISME J. 2013 Sep;7(9):1738-51. doi: 10.1038/ismej.2013.67. Epub 2013 May 2. ISME J. 2013. PMID: 23635867 Free PMC article. - Viral assemblage composition in Yellowstone acidic hot springs assessed by network analysis.
Bolduc B, Wirth JF, Mazurie A, Young MJ. Bolduc B, et al. ISME J. 2015 Oct;9(10):2162-77. doi: 10.1038/ismej.2015.28. Epub 2015 Jun 30. ISME J. 2015. PMID: 26125684 Free PMC article. - An inexpensive, accurate, and precise wet-mount method for enumerating aquatic viruses.
Cunningham BR, Brum JR, Schwenck SM, Sullivan MB, John SG. Cunningham BR, et al. Appl Environ Microbiol. 2015 May 1;81(9):2995-3000. doi: 10.1128/AEM.03642-14. Epub 2015 Feb 20. Appl Environ Microbiol. 2015. PMID: 25710369 Free PMC article. - RNA Viruses in Aquatic Ecosystems through the Lens of Ecological Genomics and Transcriptomics.
Kolundžija S, Cheng DQ, Lauro FM. Kolundžija S, et al. Viruses. 2022 Mar 28;14(4):702. doi: 10.3390/v14040702. Viruses. 2022. PMID: 35458432 Free PMC article. Review. - A Review on Viral Metagenomics in Extreme Environments.
Dávila-Ramos S, Castelán-Sánchez HG, Martínez-Ávila L, Sánchez-Carbente MDR, Peralta R, Hernández-Mendoza A, Dobson ADW, Gonzalez RA, Pastor N, Batista-García RA. Dávila-Ramos S, et al. Front Microbiol. 2019 Oct 18;10:2403. doi: 10.3389/fmicb.2019.02403. eCollection 2019. Front Microbiol. 2019. PMID: 31749771 Free PMC article. Review.
References
- Adams MH. Bacteriophages. New York, USA: Interscience Publishers; 1959.
- Anderson MA, Morel FMM. The influence of aqueous iron chemistry on the uptake of iron by the coastal diatom Thalassiosira weisflogii. Limnol Oceanogr. 1982;27:789–813.
- Bergh O, Borsheim KY, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–468. - PubMed
LinkOut - more resources
Full Text Sources