Fission of tubular endosomes triggers endosomal acidification and movement - PubMed (original) (raw)
Fission of tubular endosomes triggers endosomal acidification and movement
Kumi Mesaki et al. PLoS One. 2011.
Abstract
The early endosome acts as a sorting station for internalized molecules destined for recycling or degradation. While recycled molecules are sorted and delivered to tubular endosomes, residual compartments containing molecules to be degraded undergo "maturation" before final degradation in the lysosome. This maturation involves acidification, microtubule-dependent motility, and perinuclear localization. It is currently unknown how sorting and the processes of maturation cooperate with each other. Here, we show that fission of a tubular endosome triggers the maturation of the residual endosome, leading to degradation. Use of the dynamin inhibitor dynasore to block tubular endosome fission inhibited acidification, endosomal motility along microtubules, perinuclear localization, and degradation. However, tubular endosome fission was not affected by inhibiting endosomal acidification or by depolymerizing the microtubules. These results demonstrate that the fission of recycling tubules is the first important step in endosomal maturation and degradation in the lysosome. We believe this to be the first evidence of a cascade from sorting to degradation.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
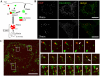
Figure 1. Recycling and degradative pathways are segregated by tubule fission.
A, Recycling and degradative pathway are illustrated, with the typical time course of intracellular transferrin and EGF in our experiments shown. Boxed words represent the marker for each compartment. EE, early endosome; LE, late endosome; RE, recycling endosome. B, HeLa cells were bound to Alexa555-EGF and Alexa488-transferrin on ice. After rinsing, the cells were incubated at 37°C for the time indicated and fixed. Scale bar, 20 µm. C, Cells were bound with ligands and transferred to 37°C as above. Live images were taken 10 min post-internalization. Frames were captured every 2 sec. Scale bar, 20 µm. (Also see Movie S1)
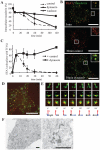
Figure 2. Dynasore inhibits transferrin recycling in early endosomes.
A, Cells were incubated with biotin-conjugated transferrin on ice for 1 h, rinsed, and transferred to 37°C. At each time point, cells were lysed and intracellular transferrin was measured by ELISA. In the ELISA assay, the cell lysates bound to plates coated with goat anti-transferrin antibodies (EY Laboratories, San Mateo, CA) and were detected with streptavidin-HRP. B, HeLa cells were incubated with Alexa 488-transferrin (Molecular Probes) on ice for 1 h, and then processed as in A. After 5 min, the media was changed to medium containing DMSO (control) or dynasore. At the time indicated, the cells were fixed and stained for EEA1 (BD Transduction Laboratories, San Jose, CA). Scale bar, 20 µm. C, Colocalization of EEA1 and transferrin was measured and is represented mean ± S.D. N = 20 cells. D, HeLa cells were bound with Alexa 555-EGF (Molecular Probes) and Alexa 488-transferrin on ice for 1 h. After rinsing, the cells were incubated at 37°C for 5 min and dynasore was added. Thirty minutes after internalization, the cells were fixed and observed. Scale bar, 20 µm. Pictures were processed for 3D reconstruction using MetaMorph software (E). F, Cells were bound to HRP-transferrin and treated for 30 min as in D. The cells were then incubated in a DAB-containing solution on ice for 30 min, fixed, and processed for electron microscopy. Scale bar, 500 nm.
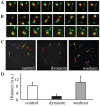
Figure 3. Dynasore inhibits tubule fission and endosomal motility.
HeLa cells were bound on ice with Alexa555-EGF and Alexa488-transferrin, rinsed, and transferred to 37°C. After 5 min, dynasore was added, and the cells were incubated further for another 25 min. For washout, the cells were rinsed at 30 min with dynasore-free media. Live images were taken just before washout (A) and at 10 min after washout (B). Frames were captured every 2 sec for a total of 2 min 30 s (See also Movie S3). C, Movements of EGF-positive endosomes for 90 s were manually tracked and are illustrated. D, The mean total movement distance of EGF-positive endosomes for 2 min. 20 endosomes were measured from 4 cells; Error bar, S.D.
Figure 4. Degradative pathway is inhibited in the early endosome.
A, HeLa cells were incubated with Alexa555-EGF on ice for 1 h, rinsed, and transferred to 37°C. After 5 min, the media was changed to DMSO- (control) or dynasore-containing media and incubated further for the time period indicated. Cells were fixed and stained using anti-EEA1 or anti-LAMP1 (Santa Cruz Biotechnology, Santa Cruz, CA) antibody. B, The cells displaying localized endosomes were manually counted. N = 80 cells. C, Cells were incubated with biotin-conjugated EGF on ice for 1 h and processed as in Figure 1A. Intracellular EGF was measured by ELISA. D, Cells were stimulated by EGF. At the indicated time after stimulation, cells were lysed and subjected to SDS-PAGE and an immunoblot analysis using an anti-EGF receptor or anti-ß-actin.
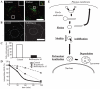
Figure 5. Acidification is required for perinuclear localization of endosomes, and a cascade model of the degradative pathway.
A, HeLa cells were bound on ice with Alexa555-EGF, rinsed, and transferred to 37°C. After 5 min, dynasore was added. For washout, after 30 min the cells were rinsed and incubated in dynasore-free media. Images are just before washout (left) and 10 min after washout (right). B, HeLa cells were incubated with Alexa555-EGF on ice for 1 h, rinsed, and transferred to 37°C. After 5 min of internalization, DMSO (left) or Baf (right) was added and the cells were further incubated. After 30 min, the cells were fixed. Dashed-line circle, nucleus; Dashed-line (not circle), outline of the cell; Scale bar, 20 µm. C, The endosomes displaying perinuclear localization were manually counted. N = 80 cells. D, Cells were incubated with biotin-conjugated EGF on ice for 1 h. After rinsing, the cells were transferred to 37°C, incubated for the time indicated, and processed by ELISA. Nocodazole was added at 1 hour before the incubation, while bafilomycin A1 was added at 5 min after internalization. E, Both internalized EGF and transferrin initially enter the same early endosomes. EGF is sorted into vacuolar domains and unsorted transferrin is collected into tubules. After tubule fission, intraluminal acidification of EGF-containing endosomes proceeds, as endosomes are motile. As a result, enlarged endosomes are recruited around the nucleus and degradation is finally completed. Dynasore inhibits tubule fission, which blocks the subsequent steps. Bafilomycin A1 inhibits endosomal acidification and causes inhibition of perinuclear localization and degradation, but not of fission or motility. Nocodazole inhibits endosomal motility, resulting in impaired perinuclear recruitment and degradation, but normal endosomal acidification.
Similar articles
- Recycling endosome tubule morphogenesis from sorting endosomes requires the kinesin motor KIF13A.
Delevoye C, Miserey-Lenkei S, Montagnac G, Gilles-Marsens F, Paul-Gilloteaux P, Giordano F, Waharte F, Marks MS, Goud B, Raposo G. Delevoye C, et al. Cell Rep. 2014 Feb 13;6(3):445-54. doi: 10.1016/j.celrep.2014.01.002. Epub 2014 Jan 23. Cell Rep. 2014. PMID: 24462287 Free PMC article. - SNX-BAR-mediated endosome tubulation is co-ordinated with endosome maturation.
van Weering JR, Verkade P, Cullen PJ. van Weering JR, et al. Traffic. 2012 Jan;13(1):94-107. doi: 10.1111/j.1600-0854.2011.01297.x. Epub 2011 Oct 31. Traffic. 2012. PMID: 21973056 - A novel live-cell imaging assay reveals regulation of endosome maturation.
Podinovskaia M, Prescianotto-Baschong C, Buser DP, Spang A. Podinovskaia M, et al. Elife. 2021 Nov 30;10:e70982. doi: 10.7554/eLife.70982. Elife. 2021. PMID: 34846303 Free PMC article. - The endosomal-lysosomal system: from acidification and cargo sorting to neurodegeneration.
Hu YB, Dammer EB, Ren RJ, Wang G. Hu YB, et al. Transl Neurodegener. 2015 Sep 30;4:18. doi: 10.1186/s40035-015-0041-1. eCollection 2015. Transl Neurodegener. 2015. PMID: 26448863 Free PMC article. Review. - The enigmatic endosome - sorting the ins and outs of endocytic trafficking.
Naslavsky N, Caplan S. Naslavsky N, et al. J Cell Sci. 2018 Jul 6;131(13):jcs216499. doi: 10.1242/jcs.216499. J Cell Sci. 2018. PMID: 29980602 Free PMC article. Review.
Cited by
- Exosome mimicry by a HAVCR1-NPC1 pathway of endosomal fusion mediates hepatitis A virus infection.
Costafreda MI, Abbasi A, Lu H, Kaplan G. Costafreda MI, et al. Nat Microbiol. 2020 Sep;5(9):1096-1106. doi: 10.1038/s41564-020-0740-y. Epub 2020 Jun 15. Nat Microbiol. 2020. PMID: 32541946 Free PMC article. - cPLA2α and EHD1 interact and regulate the vesiculation of cholesterol-rich, GPI-anchored, protein-containing endosomes.
Cai B, Caplan S, Naslavsky N. Cai B, et al. Mol Biol Cell. 2012 May;23(10):1874-88. doi: 10.1091/mbc.E11-10-0881. Epub 2012 Mar 28. Mol Biol Cell. 2012. PMID: 22456504 Free PMC article. - Centronuclear Myopathy Caused by Defective Membrane Remodelling of Dynamin 2 and BIN1 Variants.
Fujise K, Noguchi S, Takeda T. Fujise K, et al. Int J Mol Sci. 2022 Jun 3;23(11):6274. doi: 10.3390/ijms23116274. Int J Mol Sci. 2022. PMID: 35682949 Free PMC article. Review. - Dynamin Inhibitors Prevent the Establishment of the Cytomegalovirus Assembly Compartment in the Early Phase of Infection.
Štimac I, Jug Vučko N, Blagojević Zagorac G, Marcelić M, Mahmutefendić Lučin H, Lučin P. Štimac I, et al. Life (Basel). 2021 Aug 25;11(9):876. doi: 10.3390/life11090876. Life (Basel). 2021. PMID: 34575026 Free PMC article. - Sortilin limits EGFR signaling by promoting its internalization in lung cancer.
Al-Akhrass H, Naves T, Vincent F, Magnaudeix A, Durand K, Bertin F, Melloni B, Jauberteau MO, Lalloué F. Al-Akhrass H, et al. Nat Commun. 2017 Oct 30;8(1):1182. doi: 10.1038/s41467-017-01172-5. Nat Commun. 2017. PMID: 29084952 Free PMC article.
References
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. - PubMed
- Di Fiore PP, De Camilli P. Endocytosis and signaling. an inseparable partnership. Cell. 2001;106:1–4. - PubMed
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. - PubMed
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. - PubMed
- Slagsvold T, Pattni K, Malerod L, Stenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources